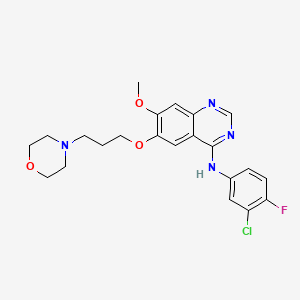
Gefitinib, 184475-35-2, Iressa, ZD1839, Irressat, N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine, gefitinib (zd1839), ZD 1839, ZD-1839, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine, gefitinibum, CCRIS 9011, UNII-S65743JHBS, Gefitinib (GMP), NSC-759856, N-(3-Chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine, S65743JHBS, DTXSID8041034, CHEBI:49668, 4-(3'-Chloro-4'-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine, MFCD04307832, CHEMBL939, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-4-quinazolinamine, DTXCID6021034, NSC715055, NSC 759856, Gefitinib [USAN], NCGC00159455-02, GEFITINIB (MART.), GEFITINIB [MART.], N-(3-chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine, 3-Chloro-4-Fluoro-N-[(4z)-7-Methoxy-6-(3-Morpholin-4-Ylpropoxy)quinazolin-4(1h)-Ylidene]aniline, C22H24ClFN4O3, GEFITINIB (EP MONOGRAPH), GEFITINIB [EP MONOGRAPH], 4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-(4-morpholinyl)propoxy)-, Iressa(TM), IRE, Iressa (TN), CAS-184475-35-2, SR-00000000262, Gefitinib (JAN/USAN/INN), Gefitinib [USAN:INN:BAN], Gefitini; Iressa, 4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-(4-morpholinyl)propoxy)-4-quinazolinamine, Iressa (AstraZeneca), nchembio866-comp14, Kinome_3321, Kinome_3322, GEFITINIB [INN], GEFITINIB [JAN], GEFITINIB [MI], GEFITINIB [VANDF], GEFITINIB [WHO-DD], SCHEMBL7866, Gefitinib,ZD-1839,Iressa, GEFITINIB [EMA EPAR], KBioSS_002241, MLS003899193, CU-00000000396-1, BDBM5447, cid_123631, GTPL4941, GEFITINIB [ORANGE BOOK], Gefitinib, >=98% (HPLC), L01XE02, BCPP000221, HMS2089B19, HMS3244M21, HMS3244M22, HMS3244N21, HMS3295A21, HMS3413H08, HMS3654A07, HMS3677H08, HMS3714A05, HMS3748E17, Pharmakon1600-01502274, BCP01365, Tox21_111683, HY-50895G, NSC759856, NSC800105, s1025, STK621310, AKOS000280752, Tox21_111683_1, AB20814, AC-1556, BCP9000718, CCG-220642, CS-0124, DB00317, KS-1204, NSC-715055, NSC-800105, 4-[(3-Chloro-4-fluorophenyl)amino]-7-methoxy-6-(3-morpholinopropoxy)quinazoline, 4-Quinazolinamine, N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-4-morpholin)propoxy)-, 6-(3-morpholinopropoxy)-N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-amine, NCGC00159455-03, NCGC00159455-04, NCGC00159455-05, NCGC00159455-06, NCGC00159455-08, NCGC00159455-09, NCGC00159455-14, BCB03_000781, BG164498, HY-50895, SMR002204119, SY002154, AM20090619, CS-0622782, FT-0602325, G0546, NS00006312, SW199108-4, D01977, EN300-123024, G-4408, K00240, AB01273954-01, AB01273954-02, AB01273954-03, AB01273954_04, A812870, Q417824, Q-201149, SR-00000000262-2, SR-00000000262-3, Gefitinib, EuropePharmacopoeia (EP) Reference Standard, Z1546610485, 4-(3'-chloro-4'-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)-quinazoline, Gefitinib for system suitability, EuropePharmacopoeia (EP) Reference Standard, (3-CHLORO-4-FLUORO-PHENYL)-[7-METHOXY-6-(3-MORPHOLIN-4-YL-PROPOXY)-QUINAZOLIN-4-YL]-AMINE