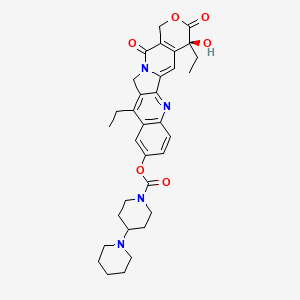
irinotecan, 97682-44-5, (+)-Irinotecan, Camptosar, Irinophore C, Irinotecanum, Biotecan, Irinotecan lactone, Irinotecan mylan, Irinotecanum [INN-Latin], Campto, Irinotecan Free base, CPT-11, (S)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl [1,4'-bipiperidine]-1'-carboxylate, HSDB 7607, Irinotecan (INN), NSC-728073, CHEBI:80630, CHEMBL481, UNII-7673326042, DTXSID1041051, 97682-44-5 (Free base), NSC728073, NSC 728073, (4S)-4,11-DIETHYL-4-HYDROXY-3,14-DIOXO-3,4,12,14-TETRAHYDRO-1H-PYRANO[3',4':6,7]INDOLIZINO[1,2-B]QUINOLIN-9-YL 1,4'-BIPIPERIDINE-1'-CARBOXYLATE, 1,4'-Bipiperidine-1'-carboxylic acid (S)-4,11-diethyl-3,4,12,14- tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl ester, IRINOTECAN [INN], Irinotecanum (INN-Latin), Irinotecan [INN:BAN], (1,4'-Bipiperidine)-1'-carboxylic acid, 4,11-diethyl-3,4,12-14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl ester, (S)-, CPT-11 hydrochloride;Camptothecin 11 hydrochloride, Irrinotecan, Biotecan (TN), (1,4'-Bipiperidine)-1'-carboxylic acid, (4S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl ester, [1,4'-Bipiperidine]-1'-carboxylic acid, (4S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl ester, 1,4'-bipiperidine-1'-carboxylic acid (s)-4,11-diethyl-3,4,12,14- tetrahydro-4-hydroxy-3,14-dioxo-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl ester, Irinotecan?, MFCD00866307, (4S)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl (1,4'-bipiperidine)-1'-carboxylate, (4S)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl [1,4'-bipiperidine]-1'-carboxylate, IRINOTECAN [MI], IRINOTECAN [HSDB], IRINOTECAN [VANDF], SCHEMBL4034, IRINOTECAN [WHO-DD], IRINOTECAN; CPT-11, BSPBio_002346, GTPL6823, DTXCID9021051, AMY4227, L01XX19, 1u65, BCP02860, BDBM50128267, s1198, AKOS015894969, AB07527, AC-7469, BCP9000793, CS-1138, DB00762, NCGC00178697-02, NCGC00178697-05, (4S)-4,11-Diethyl-4-hydroxy-3,14-dioxo-4,12-dihydro-1H-pyrano[3,4-f]quinolino[2,3-a]indolizin-9-yl 4-piperidylpiperidinecarboxylate, AS-14323, HY-16562, NCI60_005051, NS00004943, D08086, EN300-708800, AB00698464-07, AB00698464-09, AB00698464-10, AB00698464-11, AB00698464_12, AB00698464_13, AB00698464_14, A845740, Q412197, BRD-K08547377-003-02-4, (diethyl-hydroxy-dioxo-[?]yl) 4-(1-piperidyl)piperidine-1-carboxylate, (+)-7-ethyl-10-hydroxycamptothecine 10-(1,4'-bipiperidine)-1'-carboxylate, 2-methoxy-5-[2-(3-sulfophenyl)-5-(4-sulfophenyl)pyrylium-4-yl]benzenesulfonic acid, (+)-(4S)-4,11-diethyl-4-hydroxy-9-((4-piperidino-piperidino)carbonyloxy)-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinol-3,14,(4H,12H)-dione, (1,4'-bipiperidine)-1'-carboxylic acid (S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-9-yl ester, (19S)-10,19-Diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.0[2,11].0[4,9].0[15,20]]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaen-7-yl 4-(piperidin-1-yl)piperidine-1-carboxylate, (19S)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaen-7-yl [1,4'-bipiperidine]-1'-carboxylate, (19S)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaen-7-yl 4-(piperidin-1-yl)piperidine-1-carboxylate, (4S)-4,11-DIETHYL-4-HYDROXY-3,14-DIOXO-3,4,12,14-TETRAHYDRO-1H-PYRANO[3'',4'':6,7]INDOLIZINO[1,2-B]QUINOLIN-9-YL 1,4''-BIPIPERIDINE-1''-CARBOXYLATE, (S)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl[1,4'-bipiperidine]-1'-carboxylate, [(19S)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-7-yl] 4-piperidin-1-ylpiperidine-1-carboxylate, [1,4'']bipiperidinyl-1''-carboxylic acid (S)-4,11-diethyl-4-hydroxy-3,13-dioxo-3,4,12,13-tetrahydro-1H-2-oxa-6,12a-diaza-dibenzo[b,h]fluoren-9-yl ester, [1,4'']Bipiperidinyl-1''-carboxylic acid 4,11-diethyl-4-hydroxy-3,13-dioxo-3,4,12,13-tetrahydro-1H-2-oxa-6,12a-diaza-dibenzo[b,h]fluoren-9-yl ester