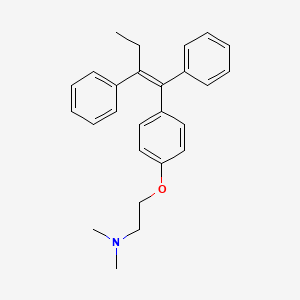
tamoxifen, 10540-29-1, trans-Tamoxifen, Crisafeno, Diemon, Tamoxifene, Citofen, Istubol, Oncomox, Retaxim, Tamoxen, Valodex, Tamoxifeno, Tamoxifenum, Novaldex, Tamoxifen (Z), Mammaton, Tamoxifene [INN-French], Tamoxifenum [INN-Latin], Tamoxifeno [INN-Spanish], Tamoplex, CCRIS 3275, (Z)-2-(4-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N-dimethylethanamine, HSDB 6782, 1-p-beta-Dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene, 1-para-beta-Dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene, EINECS 234-118-0, Tamoxifen (INN), NSC-727681, Tamoxifen (Standard), DTXSID1034187, UNII-094ZI81Y45, CHEBI:41774, 2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine, CHEMBL83, ICI-47699, Tamizam, 094ZI81Y45, (2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethyl)dimethylamine, trans-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine, (Z)-2-(para-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N-dimethylamine (IUPAC), 2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine, C26H29NO, DTXCID9014187, EC 234-118-0, Ethanamine, 2-(4-(1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethyl-, (Z)-, Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl-, (Z)-2-[4-(1,2)-DIPHENYL-1-BUTENYL)-PHENOXY]-N,N-DIMETHYLETHANAMINE, (Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene, TAMOXIFEN [INN], TAMOXIFEN (IARC), TAMOXIFEN [IARC], (Z)-2-(4-(1,2-diphenylbut-1-enyl)phenoxy)-N,N-dimethylethanamine, (Z)-2-(para-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N-dimethylamine, (z)-2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethylethanamine, Tamoxifene (INN-French), Tamoxifenum (INN-Latin), Tamoxifen [INN:BAN], Tamoxifeno (INN-Spanish), Ethylamine, N,N-dimethyl-2-(p-(1,2-diphenyl-1-butenyl)phenoxy)-, (Z)-, (E/Z)-Tamoxifen, 2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethyl-ethanamine, Tamoxifen (1.0 mg/mL in Methanol), Ethanamine, 2-(4-((1Z)-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethyl-, [3H]tamoxifen, ETHANAMINE, 2-(4-((1Z)-1,2-DIPHENYL-1-BUTEN-1-YL)PHENOXY)-N,N-DIMETHYL-, Tamoxifen (TN), [3H]-tamoxifen, Tamoplex (TN), SMR000059172, CAS-10540-29-1, TRANS FORM OF TAMOXIFEN, ICI 47699, ,citrate, Z-Tamoxifen, 2-(4-((1Z)-1,2-diphenylbut-1-en-1-yl)phenoxy)-N,N-dimethylethanamine, Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-buten-1-yl]phenoxy]-N,N-dimethyl-, Tamoxifen, 7, Tocris-0999, 1ya4, Tamoxifen, >=99%, TAMOXIFEN [MI], TAMOXIFEN [HSDB], Prestwick2_000146, Prestwick3_000146, Spectrum5_001417, Spectrum5_002043, TAMOXIFEN [VANDF], UPCMLD-DP027, SCHEMBL4084, TAMOXIFEN [WHO-DD], BIDD:PXR0003, Lopac0_001203, BSPBio_000252, BSPBio_001150, BSPBio_001982, MLS001332535, MLS001332536, BIDD:ER0008, BIDD:GT0009, Tamoxifen, analytical standard, BPBio1_000278, GTPL1016, GTPL5384, BDBM20607, cid_2733526, L02BA01, cMAP_000044, HMS1362J11, HMS1792J11, HMS1990J11, HMS2090N08, HMS2232C12, HMS3261D09, HMS3403J11, HMS3411P04, HY-13757AR, ICI47699, Tox21_201243, Tox21_300539, Tox21_500494, (Z)-2-[p-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine, 1-p-.beta.-Dimethylamino-ethoxyphenyl-trans-1,2-diphenylbut-1-ene, HB0601, HY-13757A, MFCD00010454, NSC727681, AKOS022143035, AM84324, CCG-205277, DB00675, FD12063, KS-1472, LP00494, SDCCGSBI-0051170.P007, ICI47699;Z-Tamoxifen;trans-Tamoxifen, IDI1_000258, IDI1_002170, QTL1_000079, NCGC00024928-01, NCGC00024928-03, NCGC00024928-04, NCGC00024928-05, NCGC00024928-07, NCGC00024928-08, NCGC00024928-09, NCGC00024928-10, NCGC00024928-11, NCGC00024928-12, NCGC00024928-13, NCGC00024928-14, NCGC00024928-15, NCGC00024928-16, NCGC00024928-17, NCGC00024928-18, NCGC00024928-19, NCGC00024928-39, NCGC00024928-40, NCGC00254455-01, NCGC00258795-01, NCGC00261179-01, AC-35768, BT164438, SBI-0051170.P004, CS-0694848, NS00010353, C07108, D08559, AB00053547-16, AB00053547-17, AB00053547-18, AB00053547_19, AB00053547_20, EN300-1273241, A801229, L024126, Q412178, W-108788, BRD-K93754473-001-02-9, BRD-K93754473-048-04-6, BRD-K93754473-048-05-3, BRD-K93754473-048-10-3, Tamoxifen, certified reference material, TraceCERT(R), Z2527624834, 1-p-beta-dimethylamino-ethoxyphenyl-trans-1,2-diphenylbut-1-ene, (Z)-2-(4-(1,2-Diphenyl-1-butenyl)phenoxy)phenoxy)-N,N-dimethylethanamine, (Z)-2-(4-(1,2-diphenylbut-1-en-1-yl)phenoxy)-N,N-dimethylethan-1-amine, (Z)-2-(4-(1,2-diphenylbut-1-en-1-yl)phenoxy)-N,N-dimethylethanamine, 2-[4-[(Z)-1,2-di(phenyl)but-1-enyl]phenoxy]-N,N-dimethylethanamine