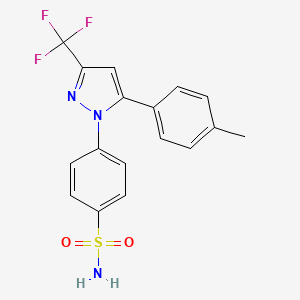
celecoxib, 169590-42-5, Celebrex, Celebra, Onsenal, Celecox, Celocoxib, 184007-95-2, Xilebao, SC-58635, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide, YM177, 4-[5-(4-METHYLPHENYL)-3-(TRIFLUOROMETHYL)-1H-PYRAZOL-1-YL]BENZENESULFONAMIDE, SC 58635, YM 177, HSDB 7038, Celecoxibum, p-(5-p-Tolyl-3-(trifluoromethyl)pyrazol-1-yl)benzenesulfonamide, UNII-JCX84Q7J1L, 194044-54-7, DFN15, JCX84Q7J1L, MFCD00941298, 4-(5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide, NSC-719627, NSC-758624, SC58635, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzene-1-sulfonamide, ELYXYB, CCRIS 9330, DFN-15, DTXSID0022777, CHEBI:41423, Celecoxib (GMP Like), YM-177, CHEMBL118, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonami de, DTXCID502777, CONSENSI COMPONENT CELECOXIB, 4-(5-(p-tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide, benzenesulfonamide, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-, NSC719627, NSC 719627, NSC 758624, Celecoxib [USAN], NCGC00091455-01, CELECOXIB (MART.), CELECOXIB [MART.], 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-benzenesulfonamide, CELECOXIB (USP-RS), CELECOXIB [USP-RS], CELECOXIB (EP MONOGRAPH), CELECOXIB [EP MONOGRAPH], CELECOXIB (USP MONOGRAPH), CELECOXIB [USP MONOGRAPH], Solexa, Benzenesulfonamide, 4-(5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)-, Benzenesulfonamide,4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-, Celebrex (TN), SMR000550473, CAS-169590-42-5, SR-01000837528, Celecixib, Celecoxib [USAN:INN:BAN], Generic Drug, ELYXYB-celecoxib, TPI-336, Celecoxib 50 mg, Celecoxib- Bio-X, Onsenal (TN), AI-525, Celecoxib 100 mg, Celecoxib 200 mg, Celecoxib 400 mg, CEP-33222, CELEBCOXIB, Spectrum_000432, 1oq5, CELECOXIB [INN], CELECOXIB [JAN], CELECOXIB [MI], CELECOXIB [HSDB], Spectrum2_001576, Spectrum3_001996, Spectrum4_000182, Spectrum5_001324, CELECOXIB [VANDF], CELECOXIB [WHO-DD], cid_2662, SCHEMBL3708, Celecoxib (JAN/USP/INN), BSPBio_003596, CELECOXIB [EMA EPAR], KBioGR_000723, KBioGR_002351, KBioSS_000912, KBioSS_002354, MLS001165684, MLS001195656, MLS001304708, MLS006011862, BIDD:GT0408, DivK1c_000893, SPECTRUM1503678, SPBio_001512, GTPL2892, CELECOXIB [ORANGE BOOK], Celecoxib, >=98% (HPLC), BDBM11639, HMS502M15, KBio1_000893, KBio2_000912, KBio2_002351, KBio2_003480, KBio2_004919, KBio2_006048, KBio2_007487, KBio3_002830, KBio3_003037, EX-A175, L01XX33, M01AH01, cMAP_000027, NINDS_000893, BCPP000290, HMS1922G14, HMS2089L18, HMS2093I07, HMS2234N18, HMS3259L08, HMS3261A14, HMS3373A09, HMS3654H09, HMS3715F11, HMS3867I03, HMS3884M07, HY-14398GL, Pharmakon1600-01503678, ALBB-033772, BCP02156, Tox21_111135, Tox21_201964, Tox21_300599, Tox21_500406, US8741944, Comparative Compound, 4-[5-(p-tolyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide, BBL029086, CCG-39354, NSC758624, s1261, STL373576, Celecoxib 1.0 mg/ml in Acetonitrile, AKOS015842517, CELECOXIB COMPONENT OF CONSENSI, Tox21_111135_1, AC-4228, AM84588, BCP9000507, CS-0570, DB00482, KS-1041, NC00708, SB19318, IDI1_000893, NCGC00091455-02, NCGC00091455-03, NCGC00091455-04, NCGC00091455-05, NCGC00091455-06, NCGC00091455-07, NCGC00091455-08, NCGC00091455-09, NCGC00091455-13, NCGC00254540-01, NCGC00259513-01, NCGC00261091-01, BC164295, BP-30217, HY-14398, NCI60_041049, PHA-00846533, SY064976, SBI-0051875.P002, CJ-016377, CP-598107, UNM-0000305813, C2816, CS-0901612, FT-0601628, FT-0623536, FT-0700357, NS00002556, SW199611-3, PF-00345549, A25046, C07589, D00567, EN300-119504, AB00052396-07, AB00052396-08, AB00052396-09, AB00052396_10, AB00052396_11, Q408801, J-010566, J-520011, Q-200816, SR-01000837528-2, SR-01000837528-3, BRD-K02637541-001-02-4, BRD-K02637541-001-06-5, Z1258938406, Celecoxib, European Pharmacopoeia (EP) Reference Standard, Celecoxib, United States Pharmacopeia (USP) Reference Standard, 4-[5-(p-Tolyl)-3-(trifluoromethyl)-1-pyrazolyl]benzenesulfonamide, 4-(5-p-tolyl-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide, 4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-pyrazol-1-yl]benzenesulfonamide, 5-(4-Methylphenyl)-1-(4-sulfamoylphenyl)-3-(trifluoromethyl)pyrazole, Celecoxib, Pharmaceutical Secondary Standard; Certified Reference Material, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyazol-1-yl]benezenesulfonamide, 4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-pyrazol-1-yl]benzenesulfonamide;4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide