2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-1-benzopyrylium, 2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-1-benzopyran-4-one, 3,3',4',7-Tetrahydroxy-flavone, 3,3',4',7-Tetrahydroxyflavone, 5-Desoxy-quercetin, fisetin, fisetinidin



| Name | Fisetin | ||
| PubChem CID | 5281614 | ||
| Molecular Weight | 286.24g/mol | ||
| Synonyms |
2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-1-benzopyrylium, 2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-1-benzopyran-4-one, 3,3',4',7-Tetrahydroxy-flavone, 3,3',4',7-Tetrahydroxyflavone, 5-Desoxy-quercetin, fisetin, fisetinidin |
||
| Formula | C₁₅H₁₀O₆ | ||
| SMILES | C1=CC(=C(C=C1C2=C(C(=O)C3=C(O2)C=C(C=C3)O)O)O)O | ||
| InChI | 1S/C15H10O6/c16-8-2-3-9-12(6-8)21-15(14(20)13(9)19)7-1-4-10(17)11(18)5-7/h1-6,16-18,20H | ||
| InChIKey | XHEFDIBZLJXQHF-UHFFFAOYSA-N | ||
| CAS Number | 528-48-3 | ||
| ChEMBL ID | CHEMBL31574 | ||
| ChEBI ID | CHEBI:42567 | ||
| Herb ID | HBIN026506 | ||
| Drug Bank ID | DB07795 | ||
| KEGG ID | C10041 | ||
| Toxicity | Organism | Test Type | Route(Dose) |
| rat | LD50 | intraperitoneal(165 mg/kg) | |
| mouse | LD50 | intraperitoneal(254 mg/kg) | |
| rat | LD50 | oral(322 mg/kg) | |
| Structure | 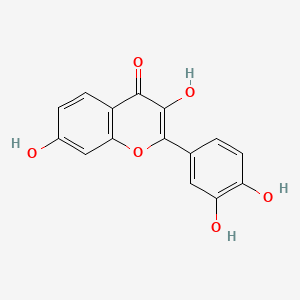
|
Download
2D
MOL
3D
MOL
|
|
| Chineses Pinyin | ErCha | ||
| Use Part | Peeled Branches And Trunks | ||
| Habitat | YunNan, GuangDong, GuangXi, FuJian | ||
| Flavor | Bitter, Astringent | ||
| Meridian Tropism | Lung, Heart | ||
| Chineses Pinyin | JiangXiang | ||
| Use Part | Dried Heartwood Of Tree Trunk And Root | ||
| Habitat | HaiNan, GuangDong, GuangXi, YunNan | ||
| Flavor | Pungent | ||
| Meridian Tropism | Liver, Spleen | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Fabales
-->Family: Fabaceae
-->Genus: Dalbergia
-->Species: Dalbergia odorifera
|
||
| Chineses Pinyin | ZaoJiaoCi | ||
| Use Part | Thorn | ||
| Habitat | JiangSu, HuBei, HeBei, ShanXi, HeNan, ShanDong, GuangDong, GuangXi, SiChuan, AnHui, ZheJiang, GuiZhou, Shaanxi, JiangXi, GanSu | ||
| Flavor | Pungent | ||
| Meridian Tropism | Liver, Stomach | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Fabales
-->Family: Fabaceae
-->Genus: Gleditsia
-->Species: Gleditsia sinensis
|
||
| Chineses Pinyin | JuanBai | ||
| Use Part | Dried Whole Herb | ||
| Habitat | China | ||
| Flavor | Pungent | ||
| Meridian Tropism | Liver, Heart | ||
| Chineses Pinyin | MangGuoYe | ||
| Use Part | Leaf | ||
| Habitat | FuJian, TaiWan, GuangDong, HaiNan, GuangXi, YunNan | ||
| Flavor | Mildly sour | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Sapindales
-->Family: Anacardiaceae
-->Genus: Mangifera
-->Species: Mangifera indica
|
||
| Chineses Pinyin | LinBeiZi | ||
| Use Part | Root, Root Cortex | ||
| Habitat | SiChuan, GuiZhou, YunNan, XiZang, ChongQing, JiangSu, ZheJiang, AnHui, FuJian, JiangXi, ShanDong, ShangHai, HeBei, HeNan | ||
| Flavor | Bitter | ||
| Meridian Tropism | Heart, Liver, Spleen | ||
| Chineses Pinyin | YeQiShuYe | ||
| Use Part | Leaf | ||
| Habitat | JiangSu, ZheJiang, AnHui, FuJian, TaiWan, JiangXi, HuBei, HuNan, GuiZhou, SiChuan | ||
| Flavor | Pungent | ||
| Meridian Tropism | Lung, Liver, Spleen, Large intestine | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Sapindales
-->Family: Anacardiaceae
-->Genus: Toxicodendron
-->Species: Toxicodendron succedaneum
|
||
| Chineses Pinyin | CaoMei | ||
| Use Part | Fruit | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Rosales
-->Family: Rosaceae
-->Genus: Fragaria
-->Species: Fragaria × ananassa
|
||
| Pair Name | Fisetin, Fluorouracil | |||
| Partner Name | Fluorouracil | |||
| Disease Info | [ICD-11: 2B91.Z] | Colorectal cancer | Investigative | |
| Biological Phenomena | Induction-->Apoptosis | |||
| Gene Regulation | Down-regulation | Expression | PIK3CA | hsa5290 |
| Down-regulation | Phosphorylation | AKT1 | hsa207 | |
| Down-regulation | Phosphorylation | MTOR | hsa2475 | |
| Up-regulation | Phosphorylation | PRKAA1 | hsa5562 | |
| Down-regulation | Phosphorylation | EIF4EBP1 | hsa1978 | |
| Down-regulation | Phosphorylation | EIF4E | KEGG ID N.A. | |
| Down-regulation | Phosphorylation | RPS6KB1 | hsa6198 | |
| Down-regulation | Expression | RPTOR | hsa57521 | |
| Down-regulation | Expression | RICTOR | hsa253260 | |
| Down-regulation | Expression | MLST8 | KEGG ID N.A. | |
| Down-regulation | Phosphorylation | AKT1S1 | hsa84335 | |
| In Vitro Model | SW480 | Colon adenocarcinoma | Homo sapiens (Human) | CVCL_0546 |
| HCT 116 | Colon carcinoma | Homo sapiens (Human) | CVCL_0291 | |
| HT-29 | Colon adenocarcinoma | Homo sapiens (Human) | CVCL_0320 | |
| In Vivo Model | Since FC13K1ApcMin/+ mice develop adenomas around 1 month of age, the treatment with fisetin and 5-FU by intraperitoneal injection was started between 26 and 30 days. | |||
| Result | Fisetin and 5-fluorouracil: Effective combination for PIK3CA-mutant colorectal cancer | |||
| Pair Name | Fisetin, Sorafenib | |||
| Partner Name | Sorafenib | |||
| Disease Info | [ICD-11: 2C30] | Melanoma | Investigative | |
| Biological Phenomena | Inhibition-->Epithelial-mesenchymal transition | |||
| Gene Regulation | Up-regulation | Expression | CDH1 | hsa999 |
| Down-regulation | Expression | CDH2 | hsa1000 | |
| Down-regulation | Expression | FN1 | hsa2335 | |
| Down-regulation | Expression | MMP2 | hsa4313 | |
| Down-regulation | Expression | MMP9 | hsa4318 | |
| Down-regulation | Expression | SNAI1 | hsa6615 | |
| Down-regulation | Expression | SNAI2 | hsa6591 | |
| Down-regulation | Expression | TWIST1 | hsa7291 | |
| Down-regulation | Expression | VIM | hsa7431 | |
| Down-regulation | Expression | ZEB1 | hsa6935 | |
| In Vitro Model | A-375 | Amelanotic melanoma | Homo sapiens (Human) | CVCL_0132 |
| SK-MEL-28 | Cutaneous melanoma | Homo sapiens (Human) | CVCL_0526 | |
| In Vivo Model | Female athymic nude mice of five weeks age were subcutaneously transplanted with A375 or SK-MEL-28 cells (2.5×10⁶ A375 cells in 50 ul DMEM + 50 ul matrigel or 5×10⁶ SK-MEL-28 cells in 50 ul RPMI + 50 ul matrigel) in each flank | |||
| Result | Our findings demonstrate that fisetin potentiates the anti-invasive and anti-metastatic effects of sorafenib. Our data suggest that fisetin may be a worthy adjuvant chemotherapy for the management of melanoma. | |||
| Pair Name | Fisetin, Sorafenib | |||
| Partner Name | Sorafenib | |||
| Disease Info | [ICD-11: 2C77.Z] | Cervical cancer | Investigative | |
| Biological Phenomena | Induction-->Mitochondria-mediated apoptosis | |||
| Gene Regulation | Up-regulation | Expression | BAX | hsa581 |
| Up-regulation | Expression | BCL2 | hsa596 | |
| Up-regulation | Expression | CASP3 | hsa836 | |
| Up-regulation | Expression | CASP8 | hsa841 | |
| Up-regulation | Expression | PARP1 | hsa142 | |
| Up-regulation | Expression | TNFRSF10B | hsa8795 | |
| In Vitro Model | HeLa | Human papillomavirus-related cervical adenocarcinoma | Homo sapiens (Human) | CVCL_0030 |
| In Vivo Model | For the in vivo experiments, 1×10⁶ HeLa cells (diluted in Matrigel) were established by subcutaneous injec tion into the animal's right flank. | |||
| Result | The combination of fisetin and sorafenib exerted better synergistic effects in vitro and in vivo than either agent used alone against human cervical cancer, and this synergism was based on apoptotic potential through a mitochondrial- and DR5-dependent caspase-8/caspase-3 signaling pathway | |||
| Pair Name | Fisetin, Sorafenib | |||
| Partner Name | Sorafenib | |||
| Disease Info | [ICD-11: 2C30] | Melanoma | Investigative | |
| Biological Phenomena | Induction-->Apoptosis | |||
| Gene Regulation | Down-regulation | Expression | AKT1 | hsa207 |
| Up-regulation | Expression | BAK1 | hsa578 | |
| Up-regulation | Expression | BAX | hsa581 | |
| Down-regulation | Expression | BCL2 | hsa596 | |
| Up-regulation | Expression | CASP3 | hsa836 | |
| Down-regulation | Expression | MAPK3 | hsa5595 | |
| Down-regulation | Expression | MCL1 | hsa4170 | |
| Down-regulation | Phosphorylation | MEK1 | hsa5604 | |
| Down-regulation | Phosphorylation | MAP2K2 | hsa5605 | |
| Down-regulation | Expression | MTOR | hsa2475 | |
| Up-regulation | Expression | PARP1 | hsa142 | |
| Down-regulation | Expression | PIK3CA | hsa5290 | |
| In Vitro Model | RPMI-7951 | Melanoma | Homo sapiens (Human) | CVCL_1666 |
| A-375 | Amelanotic melanoma | Homo sapiens (Human) | CVCL_0132 | |
| In Vivo Model | Mice were subcutaneously inoculated with 0.1ml of 2.5×10⁶ A375 cells or 5×10*6 SK-MEL-28 cells (prepared in a 50ul media + 50ul matrigel) in each flank to initiate tumor growth. | |||
| Result | Fisetin potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. | |||
| Pair Name | Fisetin, Paclitaxel | |||
| Partner Name | Paclitaxel | |||
| Disease Info | [ICD-11: 2C73] | Ovarian cancer | Investigative | |
| Biological Phenomena | Induction-->Apoptosis | |||
| Gene Regulation | Up-regulation | Expression | BAK1 | hsa578 |
| Up-regulation | Activity | CASP3 | hsa836 | |
| Down-regulation | Expression | BCL-xL | hsa598 | |
| Down-regulation | Expression | ABCG2 | hsa9429 | |
| In Vitro Model | OVCAR-3 | High grade ovarian serous adenocarcinoma | Homo sapiens (Human) | CVCL_0465 |
| Caov-3 | High grade ovarian serous adenocarcinoma | Homo sapiens (Human) | CVCL_0201 | |
| TOV-112D | Ovarian endometrioid adenocarcinoma | Homo sapiens (Human) | CVCL_3612 | |
| SW626 | Colon adenocarcinoma | Homo sapiens (Human) | CVCL_1725 | |
| Result | Our study shows that PTX-FA and Fis-FA PBM NPs directly target platinum-resistant OvCa cells, induce cytotoxic/apoptotic effects, and reverse multi-drug resistance (MDR). These findings allow us to create new clinical applications using PTX-FA and Fis-FA combination nanoparticles to treat drug-resistant cancers. | |||
| Pair Name | Fisetin, Cisplatin | |||
| Partner Name | Cisplatin | |||
| Disease Info | [ICD-11: 2C25.Z] | Lung cancer | Investigative | |
| In Vitro Model | LL/2 (LLC1) | Malignant tumors of the mouse pulmonary system | Mus musculus (Mouse) | CVCL_4358 |
| In Vivo Model | Female 6-weeks old C57BL/6J mice were used for anti-tumor evaluation. Lewis lung tumor fragments (about 30 mm3) were injected subcutaneously (s.c.) bilaterally into the flanks of the mice (day 1). | |||
| Result | The combination index between liposomal fisetin and liposomal cisplatin on 3LL cell line after 24 h of exposure showed a clear synergism: CI = 0.7 for the co loaded liposomes and CI = 0.9 for the mixture of cisplatin loaded and fisetin loaded liposomes. The co-encapsulating formulation showed in vivo efficacy against an ectopic murine model of Lewis Lung carcinoma with a probable reduction in the toxicity of cisplatin through co-encapsulation with fisetin. | |||
| No. | Title | Href |
|---|---|---|
| 1 | Co-encapsulation of fisetin and cisplatin into liposomes: Stability considerations and in vivo efficacy on lung cancer animal model. Int J Pharm. 2024 Feb 15;651:123744. doi: 10.1016/j.ijpharm.2023.123744. | Click |
| 2 | Fisetin and 5-fluorouracil: Effective combination for PIK3CA-mutant colorectal cancer. Int J Cancer. 2019 Dec 1;145(11):3022-3032. doi: 10.1002/ijc.32367. | Click |
| 3 | Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget. 2016 Jan 12;7(2):1227-41. doi: 10.18632/oncotarget.6237. | Click |
| 4 | Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumour Biol. 2016 May;37(5):6987-96. doi: 10.1007/s13277-015-4526-4. | Click |
| 5 | Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget. 2015 Sep 29;6(29):28296-311. doi: 10.18632/oncotarget.5064. | Click |
| 6 | The effect of paclitaxel- and fisetin-loaded PBM nanoparticles on apoptosis and reversal of drug resistance gene ABCG2 in ovarian cancer. J Ovarian Res. 2023 Nov 21;16(1):220. doi: 10.1186/s13048-023-01308-w. | Click |
