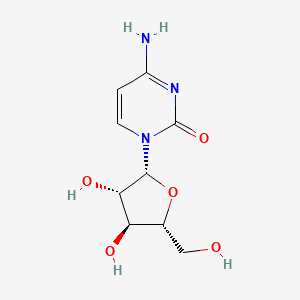
cytarabine, 147-94-4, Ara-C, Cytosine arabinoside, Aracytidine, Arabinocytidine, Cytarabinoside, Spongocytidine, Aracytin, Cytosar-U, Depocyt, Udicil, Arabinofuranosylcytosine, Arabitin, Aracytine, Cytarabina, Tarabine, Arafcyt, Erpalfa, 1-beta-D-Arabinofuranosylcytosine, arabinocytosine, Cytarabinum, Cytosine beta-D-arabinofuranoside, DepoCyte, Arabinosylcytosine, Cytarabin, Cytosar, Cytosinearabinoside, Citarabina, Depocyt (liposomal), beta-D-Arabinosylcytosine, Cytosine arabinofuranoside, Alexan, Cytosine beta-D-arabinoside, 4-Amino-1-((2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one, Cytarabinum [INN-Latin], Arabinoside C, 1beta-D-Arabinosylcytosine, Citarabina [INN-Spanish], Cytosine-1-beta-D-arabinofuranoside, AraC, Cytosine arabinose, 4-Amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinone, 1beta-Arabinofuranasylcytosine, Iretin, Cytosine 1-beta-D-arabinofuranoside, 1beta-D-Arabinofuranosylcytosine, 1-beta-D-Arabinofaranosylcytosine, beta-Arabinosylcytosine, Ara-Cytidine, U 19920A, Beta-cytosine arabinoside, Cytosine-beta-arabinoside, Cytosine-beta-D-arabinofuranoside, cytarabine liposome, 1-Arabinofuranosylcytosine, CHX 3311, CCRIS 913, U-19,920, 4-amino-1-beta-D-arabinofuranosylpyrimidin-2(1H)-one, Arabinofuranosyl Cytidine, Cytosine, 1-beta-D-arabinosyl-, 1-beta-D-Arabinosylcytosine, HSDB 3049, 1-beta-D-Arabinofuranosyl-4-amino-2(1H)pyrimidinone, EINECS 205-705-9, 1-(arabinofuranosyl)cytosine, Cyclocide, Cytosine, 1-beta-D-arabinofuranosyl-, NSC 287459, UNII-04079A1RDZ, 1-beta-arabinofuranosylcytosine, U-19920, Cytosine |A-D-Arabinofuranoside, DTXSID3022877, CHEBI:28680, AI3-52329, (beta-D-Arabinofuranosyl)cytosine, 4-Amino-1-arabinofuranosyl-2-oxo-1,2-dihydropyrimidin, 04079A1RDZ, 2(1H)-Pyrimidinone, 4-amino-1-beta-D-arabinofuranosyl-, MFCD00066487, CHEMBL803, NSC-287459, MK 8242, 4-Amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinon, 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2-dihydropyrimidin-2-one, C9H13N3O5, DTXCID702877, 2(1H)-Pyrimidinone, 4-amino-1-.beta.-D-arabinofuranosyl-, AR3, Cytonal, NCI-C04728, VYXEOS COMPONENT CYTARABINE, 1-beta-D-arabinofuranosyl-cytosine, 4-Amino-1-arabinofuranosyl-2-oxo-1,2-dihydropyrimidine, AC-1075, beta-Ara C, Cytarabine [USAN:USP:INN:BAN:JAN], NCGC00093356-03, 2(1H)-Pyrimidinone, 4-amino-1-y-D-arabinofuranosyl- [CAS], Cytarbel, (Arabinofuranosyl)cytosine, Cytarabinum (INN-Latin), Citarabina (INN-Spanish), CYTARABINE (MART.), CYTARABINE [MART.], CYTARABINE (USP-RS), CYTARABINE [USP-RS], Citozar, ara-Cytosine, Cytosine arabinoside (VAN), Tarabine PFS, CYTARABINE (EP MONOGRAPH), CYTARABINE (USP IMPURITY), CYTARABINE [EP MONOGRAPH], CYTARABINE [USP IMPURITY], Cytosine-1-beta-arabinofuranoside, CYTARABINE (USP MONOGRAPH), CYTARABINE [USP MONOGRAPH], 69-74-9, Ara-cell, Cytarabine (USAN:USP:INN:BAN:JAN), cytarabine liposome injection, Arabinosyl Cytosine, Cytosine, beta-D-arabinoside, CAS-147-94-4, SMR000449317, Depocyt (TN), Arabinoside, Cytosine, 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one, Cytartbine, Arabine, 1-.beta.-D-Arabinofuranosylcytosine, Cytarabine; 4-amino-1-beta-d-arabinofuranosylpyrimidin-2(1H)-one, SR-01000075773, NSC287459, 4-Amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinon [Czech], 4-Amino-1-arabinofuranosyl-2-oxo-1,2-dihydropyrimidin [Czech], 1-(beta-d-arabinofuranosyl)cytosine, 1-.beta.-D-arabinofuranosyl-cytosine, 4-Amino-1-b-D-arabinofuranosyl-2-(1H)-pyrimidinone, U-19920 A, cytarabine ocphosphate, 1-beta-D-Arabinofuranosylcytosine, Cytosine Arabinoside, beta -arabinosylcytosine, Arabinofuranosylcytosine?, CYTARABINE [MI], U 19920, CYTARABINE [INN], CYTARABINE [JAN], CYTARABINE [HSDB], CYTARABINE [USAN], beta -cytosine arabinoside, beta -D-arabinosylcytosine, Cytosine-beta -arabinoside, CYTARABINE [VANDF], SCHEMBL3140, 1beta -D-Arabinosylcytosine, CYTARABINE [WHO-DD], CYTARABINE [WHO-IP], BIDD:PXR0139, Lopac0_000316, MLS000758310, MLS001066340, MLS001424023, 1-beta-D-Arabinosyl-Cytosine, BIDD:GT0371, CYTARABINE [EMA EPAR], Cytosine, beta -D-arabinoside, 1beta -Arabinofuranasylcytosine, Cytarabine (JP17/USP/INN), GTPL4827, 1-ss-D-Arabinofuranosylcytosine, 2(1H)-Pyrimidinone, 4-amino-1beta-D-arabinofuranosyl-, CYTARABINE [ORANGE BOOK], SCHEMBL22591193, SCHEMBL23152019, 1beta -D-Arabinofuranosylcytosine, BETA-CYTOSINE, ARABINOSIDE, L01BC01, Cytosine, 1-beta -D-arabinosyl-, 1-beta -d-arabinofuranosylcytosine, 1-beta-D-arabinofuranosyl cytosine, Cytosine-beta -D-arabinofuranoside, HMS2051K19, HMS2090A18, HMS2230M16, HMS3713N12, 1-beta -D-Arabinofaranosylcytosine, CYTARABINE LIPOSOME [VANDF], CYTARABINUM [WHO-IP LATIN], BCP02876, Tox21_111203, Tox21_301971, BDBM50087289, CCG-51297, s1648, AKOS007930145, AKOS015896896, AM84428, Cytosine, 1-beta -D-arabinofuranosyl-, DB00987, KS-5063, NC00070, SDCCGSBI-0050304.P002, NCGC00093356-04, NCGC00093356-05, NCGC00093356-06, NCGC00093356-19, NCGC00142483-02, NCGC00255381-01, 1(BETA-D-ARABINOFURANOSYL)CYTOSINE, BA164339, HY-13605, SRI-10828-19, SRI-10828-20, SRI-10828_24, WR-28453, SL-000002, U 19,920A, C2035, NS00005949, SW197450-5, C02961, D00168, EN300-118320, 1-BETA-D-ARABINOFURANOSYLCYTOSINE; ARA-C, A808710, Q180983, SR-01000721860, J-520199, J-700005, J-700166, SR-01000075773-3, SR-01000075773-5, SR-01000721860-6, U 19,920, 1-beta -D-Arabinofuranosyl-4-amino-2(1H)pyrimidinone, 2(1H)-Pyrimidinone, 4-amino-1- -D-arabinofuranosyl, BRD-K33106058-001-07-7, BRD-K33106058-003-20-6, 1-BETA-D-ARABINOSYL-4-AMINO-2(1H)PYRIMIDINONE, 2(1H)-Pyrimidinone, 4-amino-1beta -D-arabinofuranosyl-, Cytosine -D-arabinofuranoside;Cytosine Arabinoside;Ara-C, Z1511499171, 2(1H)-Pyrimidinone, 4-amino-1-beta -D-arabinofuranosyl-, Cytarabine, European Pharmacopoeia (EP) Reference Standard, Cytarabine, United States Pharmacopeia (USP) Reference Standard, Cytosine beta-D-arabinofuranoside, crystalline, >=90% (HPLC), Cytosine beta-D-arabinofuranoside, Vetec(TM) reagent grade, 90%, Cytarabine, Pharmaceutical Secondary Standard; Certified Reference Material, 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one, Cytosine -D-arabinofuranoside hydrochloride;Cytosine Arabinoside hydrochloride;Ara-C hydrochloride