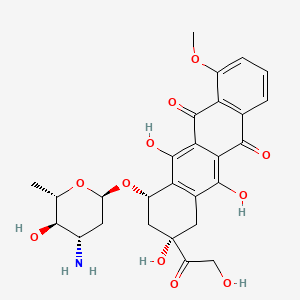
Epirubicin, Epidoxorubicin, Epiadriamycin, 4'-Epiadriamycin, 56420-45-2, 4'-epidoxorubicin, Epirubicine, Ridorubicin, Ellence, Epirubicina, Epirubicinum, Pidorubicina, Pidorubicine, Pidorubicinum, Farmorubicin, Epi-DX, 4-Epidoxorubicin, Epirubicinum [Latin], Epirubicine [INN-French], Epirubicinum [INN-Latin], Epirubicina [INN-Spanish], Pidorubicine [INN-French], Pidorubicinum [INN-Latin], Pidorubicina [INN-Spanish], 4'-epi-Doxorubicin, Pidorubicin, Epirubicin free base, WP 697, CCRIS 2261, HSDB 6962, Epirubicin (INN), NSC-256942, UNII-3Z8479ZZ5X, BRN 1445813, CHEBI:47898, 3Z8479ZZ5X, 4' Epi Adriamycin, NSC 256942, (8S,10S)-10-{[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione, DTXSID0022987, Epirubicine [French], Epirubicina [Spanish], Epirubicinum (Latin), IMI 28, 56420-45-2 (FREE BASE), EPIRUBICIN [INN], Epirubicine (INN-French), Epirubicinum (INN-Latin), Epirubicin [INN:BAN], Epirubicina (INN-Spanish), Pidorubicine (INN-French), Pidorubicinum (INN-Latin), Pidorubicina (INN-Spanish), 4'-Epi-DXR, (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranoside, 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-beta-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)-, 4' Epiadriamycin, 4' Epidoxorubicin, (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranoside, (8S,10S)-10-(((2R,4S,5R,6S)-4-Amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione, 5,12-Naphthacenedione,10-[(3-amino-2,3,6-trideoxy-a-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S,10S)-, Farmorubicin (TN), 4' Epi Doxorubicin, NSC256942, 4' Epi DXR, Epi-doxorubicin, DM6, Epi DX, Pidorubicinum (Latin), Acid, 8, EPIRUBICIN [MI], 4'-epi DX, EPIRUBICIN [HSDB], EPIRUBICIN [VANDF], CHEMBL417, SCHEMBL8582, EPIRUBICIN [WHO-DD], (7S,9S)-7-[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione, 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)-, DTXCID202987, BDBM43839, L01DB03, DM2, DB00445, NCGC00263918-04, NCGC00263918-08, (7S,9R)-7-[(2S,4S,5R,6S)-4-Amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione, (7S,9S)-7-[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione, 10-((3-amino-2,3,6-trideoxy-beta-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-(8S-cis)-5,12-naphthacenedione, HY-13624, SBI-0206890.P001, NS00069619, D07901, AB00698552-11, AB00698552-13, AB00698552-14, AB00698552_15, AB00698552_16, EN300-7410311, A831042, Q425122, BRD-K04548931-003-16-5, (1S,3S)-3-GLYCOLOYL-1,2,3,4,6,11-HEXAHYDRO-3,5,12-TRIHYDROXY-10-METHOXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-ARABINO-HEXOPYRANOSIDE, (1S,3S)-3-GLYCOLOYL-1,2,3,4,6,11-HEXAHYDRO-3,5,12-TRIHYDROXY-10-METHOXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-alpha-L-ARABINO-HEXOPYRANOSIDE, (7S,9S)-7-[(2R,4S,5R,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-4-methoxy-6,9,11-tris(oxidanyl)-9-(2-oxidanylethanoyl)-8,10-dihydro-7H-tetracene-5,12-dione, (7S,9S)-7-[[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-9-(2-hydroxy-1-oxoethyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione, (8S-cis)-10-((3-Amino-2,3,6-trideoxy-beta-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-5,12-naphthacenedione, 3-Glycoloyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl-3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranoside, 5,12-NAPHTHACENEDIONE, 10-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-ARABINO-HEXOPYRANOSYL)OXY)-7,8,9,10-TETRAHYDRO-6,8,11-TRIHYDROXY-8-(HYDROXYACETYL)-1-METHOXY-, (8S-CIS)-, 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-beta-L-arabino-hexopyranosyl)oxy-7,8,9,10-tetrahydro -6,8,(8S-cis)-, 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-.alpha.-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)-