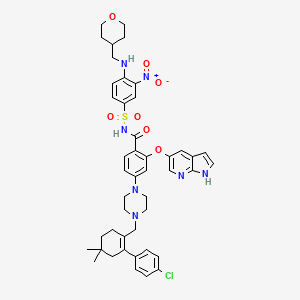
Venetoclax, 1257044-40-8, ABT-199, Venclexta, GDC-0199, ABT199, ABT 199, venclyxto, RG7601, UNII-N54AIC43PW, GDC 0199, RG-7601, Venetoclax (ABT199), N54AIC43PW, 4-[4-[[2-(4-chlorophenyl)-4,4-dimethylcyclohexen-1-yl]methyl]piperazin-1-yl]-N-[3-nitro-4-(oxan-4-ylmethylamino)phenyl]sulfonyl-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide, Venetoclax; Abt-199, Venetoclax (ABT-199), ABT-199 (GDC-0199), CHEBI:133021, DTXSID30154863, 4-(4-((2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-((tetrahydro-2H-pyran-4-ylmethyl)amino)phenyl)sulfonyl)-2-(1H-pyrrolo(2,3-b)pyridin-5-yloxy)benzamide, benzamide, 4-(4-((2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexen-1-yl)methyl)-1-piperazinyl)-n-((3-nitro-4-(((tetrahydro-2h-pyran-4-yl)methyl)amino)phenyl)sulfonyl)-2-(1h-pyrrolo(2,3-b)pyridin-5-yloxy)-, ABT 199 (Venetoclax), 2-(1H-Pyrrolo[2,3-b]pyridin-5-yloxy)-4-(4-((2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-enyl)methyl)piperazin-1-yl)-N-(3-nitro-4-((tetrahydro-2H-pyran-4-yl)methy, 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(oxan-4-ylmethyl)amino]benzene}sulfonyl)-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzamide, Benzamide, 4-[4-[[2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]-N-[[3-nitro-4-[[(tetrahydro-2H-pyran-4-yl)methyl]amino]phenyl]sulfonyl]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)-, BDBM189459, ABT 199 (>99%)(Venetoclax), 2-((1H-Pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-(((tetrahydro-2H-pyran-4-yl)methyl)amino)phenyl)sulfonyl)benzamide, 4-(4-((2-(4-CHLOROPHENYL)-4,4-DIMETHYLCYCLOHEX-1-EN-1-YL)METHYL)PIPERAZIN-1-YL)-N-((3-NITRO-4-((TETRAHYDRO-2HPYRAN-4-YLMETHYL) AMINO)PHENYL)SULFONYL)-2-(1H-PYRROLO(2,3-B)PYRIDIN-5-YLOXY)BENZAMIDE, 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-n-({3-nitro-4-[(tetrahydro-2h-pyran-4-ylmethyl)amino]phenyl}sulfonyl)-2-(1h-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide, 4-{4-[(4'-chloro-5,5-dimethyl[3,4,5,6-tetrahydro[1,1'-biphenyl]]-2-yl)methyl]piperazin-1-yl}-N-(3-nitro-4-{[(oxan-4-yl)methyl]amino}benzene-1-sulfonyl)-2-[(1H-pyrrolo[2,3-b]pyridin-5-yl)oxy]benzamide, Venetoclax [USAN:INN], venetoclaxum, Venclexta (TN), 4-(4-((4'-chloro-5,5-dimethyl(3,4,5,6-tetrahydro(1,1'-biphenyl))-2-yl)methyl)piperazin-1-yl)-N-(3-nitro-4-(((oxan-4-yl)methyl)amino)benzene-1-sulfonyl)-2-((1H-pyrrolo(2,3-b)pyridin-5-yl)oxy)benzamide, VENETOCLAX [MI], Venetoclax(ABT-199), VENETOCLAX [INN], VENETOCLAX [JAN], VENETOCLAX [USAN], Venetoclax(ABT-199)?, VENETOCLAX [WHO-DD], MLS006010298, SCHEMBL523816, Venetoclax (JAN/USAN/INN), AMY343, GTPL8318, CHEMBL3137309, DTXCID0077354, SCHEMBL19236295, VENETOCLAX [ORANGE BOOK], BDBM60828, EX-A168, L01XX52, HMS3653J06, HMS3745E07, ABT-0199, BCP06811, BDBM50162774, MFCD23160052, NSC766270, AKOS025289539, CCG-270543, CS-1155, DB11581, KS-1470, NSC-766270, SB16499, NCGC00345789-01, NCGC00345789-05, NCGC00345789-10, NCGC00345789-11, AC-28754, DA-35360, HY-15531, SMR004701366, FT-0699586, NS00073446, S8048, SW219672-1, D10679, US9174982, 5, EN300-7399830, A850921, US9174982, 369, J-005269, Q23671272, Z2037279542, )methyl]amino}phenyl)sulfonyl]-2-[(1H-pyrrolo[2,3-b]pyridin-5-yl)oxy]benzamide, GDC-0199 , ABT 199 , ABT-199 , ABT199, 4-(4-((2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-(((oxan-4-yl)methyl)amino)phenyl)sulfonyl)-2-((1H-pyrrolo(2,3-b)pyridin-5-yl)oxy)benzamide, 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-(3-nitro-4-{[(oxan-4-yl)methyl]amino}benzenesulfonyl)-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzamide, 4-[4-[[2-(4-chlorophenyl)-4,4-dimethyl-cyclohexen-1-yl]methyl]piperazin-1-yl]-N-[3-nitro-4-(tetrahydropyran-4-ylmethylamino)phenyl]sulfonyl-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide, 4-{4-[(4'-chloro-5,5-dimethyl[3,4,5,6-tetrahydro[1,1'-biphenyl]]-2-yl)methyl]piperazin-1-yl}-N-[(3-nitro-4-{[(oxan-4-yl, 4-{4-[(4'-chloro-5,5-dimethyl[3,4,5,6-tetrahydro[1,1'-biphenyl]]-2-yl)methyl]piperazin-1-yl}-N-[(3-nitro-4-{[(oxan-4-yl)methyl]amino}phenyl)sulfonyl]-2-[(1H-pyrrolo[2,3-b]pyridin-5-yl)oxy]benzamide, ABT-199; 2-(1H-Pyrrolo[2,3-b]pyridin-5-yloxy)-4-(4-((2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-enyl)methyl)piperazin-1-yl)-N-(3-nitro-4-((tetrahydro-2H-pyran-4-yl)methy