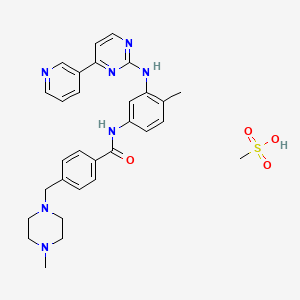
Imatinib mesylate, 220127-57-1, Gleevec, Glivec, Imatinib mesilate, imatinib methanesulfonate, Imatinib Mesylate (STI571), sti-571, Imatinib accord, Imatinib medac, Imatinib (mesylate), imatinib monomesylate, Shantinib, Imatinib (as mesilate), NSC-716051, QTI-571, Imatinib methane sulfonate, CGP 57148B, Gleevec (Imatinib mesylate), QTI571, STI 571, 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-benzamide monomethanesulfonate, DTXSID9040502, CHEBI:31690, HSDB 7142, Imatinib Methansulfonate, 8A1O1M485B, 220127-57-1 (mesylate), Imatinib mesylate [USAN], Imatinib monomethanesulfonate, UNII-8A1O1M485B, N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide methanesulfonate, DTXCID7020502, MFCD04307699, NSC716051, NSC 716051, N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide methanesulfonate, Imatinib mesilate (JAN), Imatinib mesylate (USAN), 4-[(4-methyl-1-piperazinyl)methyl]-n-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide methanesulfonate, Benzamide, 4-((4-methyl-1-piperazinyl)methyl)-N-(4-methyl-3-((4-(3-pyridinyl)-2-pyrimidinyl)amino)phenyl)-, monomethanesulfonate, Benzamide, 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-, methanesulfonate (1:1), IMATINIB MESILATE [JAN], IMATINIB MESILATE (MART.), IMATINIB MESILATE [MART.], CGP-57148B, IMATINIB MESILATE (EP MONOGRAPH), IMATINIB MESILATE [EP MONOGRAPH], CAS-220127-57-1, CGP-57148, NCGC00159456-02, Genfatinib, Gleevac, ImatinibMesylate, Imatinib, methanesulfonate salt, Imatinib mesylate?, Mesylate, Imatinib, 4-((4-methylpiperazin-1-yl)methyl)-N-(4-methyl-3-((4-pyridin-3-ylpyrimidin-2-yl)amino)phenyl)benzamide methanesulfonate, 4-[(4-methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide methanesulfonate, 4-[(4-Methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide methanesulfonate, 4-[(4-Methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[[4-(pyridin-3-yl)pyrimidin-2-yl]amino]phenyl]benzamide methanesulfonate, 4-[(4-methylpiperazin-1-yl)methyl]-N-{4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl}benzamide methanesulfonate, Gleevec (TN), Glivec (TN), ST-1571 Mesylate, Imatinib mesylate400 mg, Methanesulfonate, Imatinib, SCHEMBL8217, CHEMBL1642, Benzamide,monomethanesulfonate, Imatinib methanesulfonate salt, MLS001401456, C29H31N7O.CH4O3S, IMATINIB MESYLATE [HSDB], CGP57148B, EX-A954, IMATINIB MESYLATE [VANDF], YLMAHDNUQAMNNX-UHFFFAOYSA-N, BCPP000204, GGP-57148B, HMS2052B09, HMS2233D16, HMS3265E01, HMS3265E02, HMS3265F01, HMS3265F02, HMS3372O12, HMS3394B09, HMS3654C07, IMATINIB MESILATE [WHO-DD], BCP01255, Tox21_111684, AC-525, HB1943, s1026, IMATINIB METHANESULFONATE [MI], AKOS015852497, Tox21_111684_1, BCP9000776, CCG-101175, IMATINIB MESYLATE [ORANGE BOOK], KS-1236, NC00425, NCGC00159456-11, 111GE005, BI164678, HY-50946, methanesulfonic acid; 4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide, SMR000469175, SY013513, AM20080900, FT-0601612, I0936, NS00076459, SW197805-4, D01441, Imatinib Mesylate (CGP-57148B, STI-571), M06311, A815828, A846640, J-523068, Q-201232, Q27114666, Imatinib Mesylate,Gleevec,Glivec,CGP-57148B,STI-571, ST-1571 Mesylate , STI-571 , CGP-57148B, ;4-[(4-Methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide methanesulfonate, 4-((4-Methyl-1-piperazinyl)methyl)-N-(4-methyl-3-((4-(3-pyridinyl)-2-pyrimidinyl)amino)phenyl)benzamide Monomethanesulfonate, 4-(4-Methyl-piperazin-1-ylmethyl)-N-[4-methyl-3-(4-pyridin- 3-yl)-pyrimidin-2-ylamino)-phenyl]-benzamidemethanesulfonic acid salt, 4-(4-Methylpiperazin-1-ylmethyl)-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)phenyl]benzamide methanesulfonic acid salt, 4-[(4-Methyl-1-piperazinyl)-methyl]-N-{4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]-amino]-phenyl}-benzamide monomethanesulphonate, 4-[(4-methyl-1-piperazinyl)methyl]-n-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate, BENZAMIDE, 4-((4-METHYL-1-PIPERAZINYL)METHYL)-N-(4-METHYL-3-((4-(3-PYRIDINYL)-2-PYRIMIDINYL)AMINOPHENYL)-, METHANESULFONATE SALT, BENZAMIDE, 4-((4-METHYL-1-PIPERAZINYL)METHYL)-N-(4-METHYL-3-((4-(3-PYRIDINYL)-2-PYRIMIDINYL)AMINOPHENYL)-, METHANESULPHONATE SALT, hydron;methanesulfonate;4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide, methanesulfonic acid; 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide, N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamidemethanesulfonate