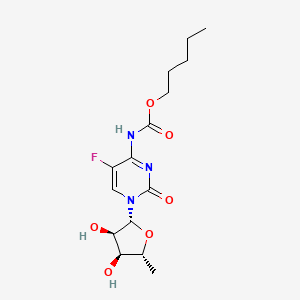
CAPECITABINE, 154361-50-9, Xeloda, Capiibine, Capecitibine, Captabin, Capecytabine, 5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine, Caxeta, Xabine, Capecitabine Sun, Capecitabine Teva, Capecitabine Medac, Capecitabine Accord, Ro 09-1978, capecitabina, capecitabinum, Capecitabin, Ecansya, Ro 09-1978/000, Pentyl (1-((2R,3R,4S,5R)-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl)carbamate, N(4)-Pentyloxycarbonyl-5'-deoxy-5-fluorocytidine, UNII-6804DJ8Z9U, Pentyl 1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinecarbamate, 5'-Deoxy-5-fluoro-N-((pentyloxy)carbonyl)cytidine, DTXSID3046451, CHEBI:31348, HSDB 7656, Ro-091978000, Cytidine, 5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]-, 6804DJ8Z9U, NSC-759853, (1-(5-Deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-carbamic acid pentyl ester, Carbamic acid, (1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-, pentyl ester, pentyl N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate, DTXCID1026451, RO-09-1978000, XELIRI COMPONENT CAPECITIBINE, Capecitabine [USAN:USP:INN:BAN], NSC 759853, Capecitabine [USAN], Ro-09-1978-000, Pentyl [1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]carbamate, CAPECITABINE (MART.), CAPECITABINE [MART.], pentyl 1-((2R,3R,4S,5R)-3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-ylcarbamate, CAPECITABINE (USP-RS), CAPECITABINE [USP-RS], Cytidine, 5'-deoxy-5-fluoro-N-((pentyloxy)carbonyl)-, CAPECITABINE (EP MONOGRAPH), CAPECITABINE (USP IMPURITY), CAPECITABINE [EP MONOGRAPH], CAPECITABINE [USP IMPURITY], Capecitabine (USAN:USP:INN:BAN), CAPECITABINE (USP MONOGRAPH), CAPECITABINE [USP MONOGRAPH], Capecitabine (Xeloda), SMR002530052, Xeloda (TN), CAS-154361-50-9, R340, NSC712807, NCGC00164569-01, Pentyl (1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl)carbamate, Capecitabine 150mg, Capecitabine 500mg, RG-340, Capecitabine- Bio-X, Ro-09-1978, CAPECITABINE [MI], R-340, CAPECITABINE [INN], CAPECITABINE [JAN], CAPECITABINE [HSDB], Xelox component capecitabine, SCHEMBL8153, CAPECITABINE [VANDF], CHEMBL1773, MLS003915642, MLS004774137, pentyl N-{1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl}carbamate, CAPECITABINE [WHO-DD], Capecitabine (JAN/USP/INN), Capecitabine Tablets, 150 mg, Capecitabine Tablets, 500 mg, GTPL6799, CAPECITABINE [EMA EPAR], Capecitabine, analytical standard, Capecitabine, >=98% (HPLC), EX-A835, L01BC06, BCPP000300, CAPECITABINE [ORANGE BOOK], GLXC-03327, HY-B0016, Tox21_112198, s1156, AKOS015920130, Tox21_112198_1, AM84502, BCP9000483, BS-1000, CCG-264841, CS-0768, DB01101, NCGC00164569-02, NCGC00164569-05, NCGC00164569-11, BC164277, BP-58647, NS00000464, D01223, n4-pentyloxycarbonyl-5'-deoxy-5-fluorocytidine, AB01274776-01, AB01274776-02, AB01274776_04, 5'-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine, Q420207, SR-01000931255, 5'-Deoxy-5-fluoro-N-((pentyloxy)carbonyl)-cytidine, J-700154, Q-200788, SR-01000931255-3, BRD-K61192372-001-08-9, Z1501480421, Capecitabine, European Pharmacopoeia (EP) Reference Standard, Capecitabine, United States Pharmacopeia (USP) Reference Standard, Capecitabine, Pharmaceutical Secondary Standard; Certified Reference Material, CARBAMIC ACID, (1-(5-DEOXY-.BETA.-D-RIBOFURANOSYL)-5-FLUORO-1,2-DIHYDRO-2-OXO-4-PYRIMIDINYL)-, PENTYL ESTER, PENTYL 1-(5-DEOXY-.BETA.-D-RIBOFURANOSYL)-5-FLUORO-1,2-DIHYDRO-2-OXO-4-PYRIMIDINECARBAMATE