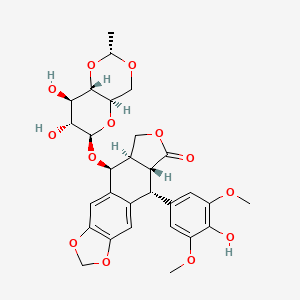
etoposide, VePesid, 33419-42-0, Toposar, trans-Etoposide, (-)-Etoposide, Lastet, Zuyeyidal, Etoposidum, Etoposido, Etoposidum [INN-Latin], VP-16, Etoposide (VP16), VP 16 (pharmaceutical), Sintopozid, Vepesid J, VP-16-213, VP 16-213, Etoposido [INN-Spanish], 4-Demethylepipodophyllotoxin beta-D-ethylideneglucoside, NK 171, CCRIS 2392, NSC-141540, DTXSID5023035, HSDB 6517, UNII-6PLQ3CP4P3, EINECS 251-509-1, 6PLQ3CP4P3, 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside), EPEG, CHEBI:4911, Epipodophyllotoxin VP-16213, Etoposide (VP-16), VP 16, 4'-O-Demethyl-1-O-(4,6-O-ethylidene-beta-D-glucopyranosyl)epipodophyllotoxin, CHEMBL44657, Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-beta-D-glucopyranoside), Epipodophyllotoxin-beta-D-ethyliden-glucoside, 4'-demethyl-, Eposide, DTXCID601473876, Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside, 4'-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-beta-D-glucopyranoside), NSC 141540, Etoposide [USAN:USP:INN:BAN:JAN], Etosid, ETOPOSIDE (IARC), ETOPOSIDE [IARC], Etoposidum (INN-Latin), Etoposido (INN-Spanish), ETOPOSIDE (MART.), ETOPOSIDE [MART.], ETOPOSIDE (USP-RS), ETOPOSIDE [USP-RS], (5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one, (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside, Etopophos (phosphate salt), ETOPOSIDE (EP IMPURITY), ETOPOSIDE [EP IMPURITY], ETOPOSIDE (EP MONOGRAPH), ETOPOSIDE (USP IMPURITY), ETOPOSIDE [EP MONOGRAPH], ETOPOSIDE [USP IMPURITY], ETOPOSIDE (USP MONOGRAPH), ETOPOSIDE [USP MONOGRAPH], Etopol, Prestwick 211, Demethylepipodophyllotoxin-beta-D-ethylideneglucoside, VP 16213, MFCD00869325, NSC141540, Etoposide (USAN:USP:INN:BAN:JAN), (10R,11R,15R,16S)-16-{[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-hexahydro-2H-pyrano[3,2-d][1,3]dioxin-6-yl]oxy}-10-(4-hydroxy-3,5-dimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0^{3,7}.0^{11,15}]hexadeca-1(9),2,7-trien-12-one, 9-((4,6-O-Ethylidine-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,4-dimethyloxyphenyl)furo(3',4'':6,7)naptho-(2,3-d)-1,3-dioxol-6(5aH)-one, SMR000112002, VePESID (TN), 4'-Demethylepipodophyllotoxin ethylidene-.beta.-D-glucoside, Demethyl Epipodophyllotoxin Ethylidine Glucoside, Etoposide,(S), NCGC00016821-01, (5R,5aR,8aR,9S)-9-(((4aR,6R,7R,8R,8aS)-7,8-Dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8H)-one, (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol -5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside, EVP, Furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, 9-[[4,6-O-(1R)-ethylidene-.beta.-D-glucopyranosyl]oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)-, CAS-33419-42-0, Etoposide; VP-16, CPD000112002, ETOPOSIDE [INN], ETOPOSIDE [JAN], ETOPOSIDE [MI], ETOPOSIDE [HSDB], ETOPOSIDE [USAN], Prestwick3_000396, ETOPOSIDE [VANDF], 4'-Demethylepipodophyllotoxin ethylidene-beta-D-glucoside, ETOPOSIDE [WHO-DD], ETOPOSIDE [WHO-IP], SCHEMBL4259, BSPBio_000611, 9-((4,6-O-Ethylidene-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one, (5R-(5alpha,5abeta,8aalpha,9beta(R*)))-, 9-((4,6-O-Ethylidine-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4- hydroxy-3,4-dimethyloxyphenyl)furo (3',4'':6,7) naptho-(2,3-d)-1,3-dioxol-6 (5aH)-one, MLS000049957, MLS001074951, MLS001424283, MLS002153463, MLS002207239, MLS002222184, Etoposide (JP17/USP/INN), BPBio1_000673, GTPL6815, ETOPOSIDE [ORANGE BOOK], L01CB01, ETOPOSIDUM [WHO-IP LATIN], HMS2052N05, HMS2089F14, HMS2096O13, HMS2232L03, HMS3713O13, (5R,5aR,8aR,9S)-9-[[4,6-O-(1R)-Ethylidene-beta-D-glucopyranosyl]oxy]-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)furo[3',4':6,7]naphtho[2,3-d]-1,3-dioxol-6(5aH)-one, EPE, EX-A1207, Tox21_110630, Tox21_302201, BDBM50127140, s1225, Etoposide - CAS 33419-42-0, AKOS007930275, BCP9000669, CCG-101165, CS-1774, DB00773, Etoposide, synthetic, >=98%, powder, NC00415, SDCCGSBI-0050405.P002, 4'-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside, NCGC00179504-02, NCGC00255126-01, AS-35312, BE164434, Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one, 9-((4,6-O-(1R)-ethylidene-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl)-, (5R,5aR,8aR,9S)-, Furo(3',4':6,7)naphtho(2,3-d)-1,3-dioxol-6(5aH)-one-, 9-((4,6-O-ethylidene-beta-D-glucopyranosyl)oxy)5,8,8a,9-tetrahydro-5-(4-hydroxy-3,5-dimethoxyphenyl), (5R-(5alpha,5abeta,8aalpha,9beta(R*)))-, HY-13629, ETOPOSIDE IMPURITY C (EP IMPURITY), SBI-0051910.P002, AB00438905, FT-0630571, NS00009585, EN300-97099, C01576, D00125, AB00438905-17, AB00438905-18, AB00438905_19, Q418817, SR-01000763196, SR-01000763196-3, BRD-K37798499-001-02-5, BRD-K37798499-001-05-8, BRD-K37798499-001-10-8, BRD-K37798499-001-14-0, BRD-K37798499-001-27-2, -5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside, Etoposide, British Pharmacopoeia (BP) Reference Standard, Z1304065033, Etoposide, European Pharmacopoeia (EP) Reference Standard, Etoposide, United States Pharmacopeia (USP) Reference Standard, 4''-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside), 4'-DEMETHYLEPIPODOPHYLLOTOXIN 9-(4,6-O-(R)-ETHYLIDENE-.BETA.-D-GLUCOPYRANOSIDE), Etoposide for system suitability, European Pharmacopoeia (EP) Reference Standard, (5R,5AR,8aR,9S)-9-(((2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahy, (5R,5aR,8aR,9S)-9-(((2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-5-(4-hydroxy-3,5-dimethoxyphenyl)-5,5a,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(8H)-one, (5R,5AR,8AR,9S)-9-((4,6-O-((1R)-ETHANE-1,1-DIYL)-.ALPHA.-D-GLUCOPYRANOSYL)OXY)-5-(4-HYDROXY-3,5-DIMETHOXYPHENYL)-5,8,8A,9-TETRAHYDRO(2)BENZOFURO(5,6-F)(1,3)BENZODIOXOL-6(5AH)-ONE, (5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxy-phenyl)-5a,6,8a,9-tetrahydro-5H-isobenzofuro[5,6-f][1,3]benzodioxol-8-one, (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-dimethoxyphenyl)-8-oxo-5,5a,6,8,8a,9-hexahydrofuro[3'',4'':6,7]naphtho[2,3-d][1,3]dioxol-5-yl 4,6-O-[(1R)-ethylidene]-beta-D-glucopyranoside, 121471-01-0, 9-((4,6-O-Ethylidine-beta-D-glucopyranosyl)oxy)-5,8,8a,9-tetrahydro-5-(4-hydroxy-3,4-dimethyloxyphenyl)furo(3'',4'''':6,7)naptho-(2,3-d)-1,3-dioxol-6(5aH)-one, FURO(3',4':6,7)NAPHTHO(2,3-D)-1,3-DIOXOL-6(5AH)-ONE-, 9-((4,6-O-ETHYLIDENE-.BETA.-D-GLUCOPYRANOSYL)OXY)5,8,8A,9-TETRAHYDRO-5-(4-HYDROXY-3,5-DIMETHOXYPHENYL), (5R-(5.ALPHA.,5A.BETA.,8A.ALPHA.,9.BETA.(R*)))-