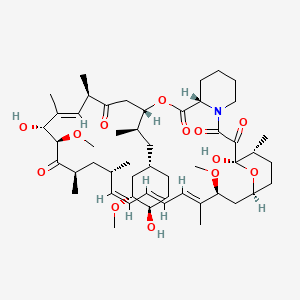
(-)-Rapamycin, 53123-88-9, AY 22989, AY-22989, I 2190A, I-2190A, I2190A, NSC 226080, RAPA, Rapammune, Rapamune, Rapamycin, SIIA 9268A, Sirolimus, Wy 090217, rapalimus, Supralimus, WY-090217, Rapamycin (Sirolimus), HYFTOR, sirolimusum, Cypher, Antibiotic AY 22989, Rapamycin (GMP), FYARRO, CCRIS 9024, CHEBI:9168, SILA 9268A, W36ZG6FT64, HSDB 7284, L04AA10, Npc-12g, Rapamycin (GMP Like), SM-88 COMPONENT SIROLIMUS, DE-109, NSC-226080, UNII-W36ZG6FT64, DTXSID5023582, MFCD00867594, Rapamycin Immunosuppressant Drug, NAB-RAPAMYCIN COMPONENT RAPAMYCIN, Rapamycin (>85%), SIROLIMUS (MART.), SIROLIMUS [MART.], EC 610-965-5, SIROLIMUS (USP-RS), SIROLIMUS [USP-RS], NSC226080, (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-{(2S)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.0(4,9)]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-3-{(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone, EVEROLIMUS IMPURITY A (EP IMPURITY), EVEROLIMUS IMPURITY A [EP IMPURITY], (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-((1R)-2-((1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl)-1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone, DTXCID503582, Perceiva, 1fkb, 1pbk, NCGC00021305-05, Rapamycin/Sirolimus, LCP-Siro, (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-((2S)-1-((1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl)propan-2-yl)-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo(30.3.1.0(4,9))hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-3-((2R)-1-((1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl)propan-2-yl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3H-23,27-epoxypyrido(2,1-c)(1,4)oxazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-3-{(1R)-2-[(1S,3R,4R)-4-hydroxy-3-(methyloxy)cyclohexyl]-1-methylethyl}-6,8,12,14,20,26-hexamethyl-10,21-bis(methyloxy)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(6H,31H)-pentone, RAP, RPM, S1039, SIROLIMUS [INN], SIROLIMUS [JAN], RAPAMYCIN [MI], Human Blood - Sirolimus, SIROLIMUS [HSDB], SIROLIMUS [USAN], SIROLIMUS [VANDF], BiomolKI2_000084, SCHEMBL3463, SIROLIMUS [WHO-DD], Rapamycin,Sirolimus,Rapamune, BIDD:PXR0165, SIROLIMUS [EMA EPAR], 23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine, MLS006010168, Sirolimus [USAN:INN:BAN], GTPL6031, SIROLIMUS [ORANGE BOOK], BDBM36609, MS-R001, S01XA23, HMS2089A21, HMS3403F11, HMS3884C03, HY-10219GL, EX-A1044, AC-722, BDBM50064359, HY-10219G, STL570275, AKOS015850976, AKOS015961618, CCG-100684, CS-0063, DB00877, NCGC00021305-06, NCGC00021305-07, Rapamycin from Streptomyces hygroscopicus, AS-11687, HY-10219, SMR004701276, Fyarro (sirolimus albumin-bound particles), UNM-0000358684, A-275, CS-0626126, CS-1010374, R0097, Rapamycin, VETRANAL(TM), analytical standard, M02444, Q32089, Q-201659, BRD-K84937637-001-04-0, BRD-K84937637-001-06-5, BRD-K89626439-001-01-0, Z2568665260, 24,25,26,27,32,33,34,34a-hexadecahydro-3H-23,27-epoxypy, Rapamycin from Streptomyces hygroscopicus, >=95% (HPLC), powder, Rapamycin from Streptomyces hygroscopicus, Vetec(TM) reagent grade, >=95%, (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-Dihydroxy-12-((1R)-2-((1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl)-1-methylethyl)-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo(30.3.1.04,9)hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone, (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-Dihydroxy-12-{(2S)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone, (3 S ,6 R ,7 E ,9 R ,10 R ,12 R ,14 S ,15 E ,17 E , 19 E ,21 S ,23 S ,26 R ,27 R ,34a S )-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34, 34a-hexadecahydro-9,27-dihydroxy-3-, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34 aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hex adecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydro xy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy- 6,8,12,14,20,26-hexamethyl-23,27-ep, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-4,9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-heptadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-223,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(6H,31H)-pentone, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-ep, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-3-{(1R)-2-[(1S,3R,4R)-4-hydroxy-3-(methyloxy)cyclohexyl]-1-methylethyl}-6,8,12,14,20,26-hexamethyl-10,21-bis(methyloxy)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-3-{(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone, (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-3-{1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-9,10,12,13,14,21,22,23,, 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone (Rapamycin), 9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-Hexadecahydro-9,27-dihydroxy-3-((1R)-2-((1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl)-1-methylethyl)-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,2