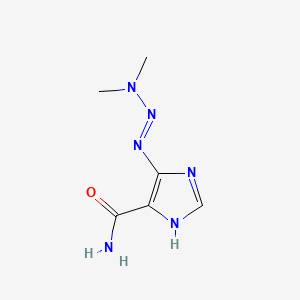
dacarbazine, 4342-03-4, DTIC, DTIC-Dome, Deticene, Biocarbazine R, Biocarbazine, Decarbazine, ICDMT, ICDT, Dacarbazinum, Dacarbasine, Dicarbazine, 5-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide, 5-(3,3-Dimethyltriazeno)imidazole-4-carboxamide, Imidazole carboxamide, NSC-45388, NSC45388, (Dimethyltriazeno)imidazolecarboxamide, 5-(Dimethyltriazeno)imidazole-4-carboxamide, Di-me-triazenoimidazolecarboxamide, NCI-C04717, 4-(3,3-Dimethyl-1-triazeno)imidazole-5-carboxamide, 7GR28W0FJI, NSC 45388, DTIE, 1H-imidazole-4-carboxamide, 5-(3,3-dimethyl-1-triazenyl)-, 4-(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide, 4-(Dimethyltriazeno)imidazole-5-carboxamide, NCI C04717, Dacarbazinum [INN-Latin], Dacarbazino [INN-Spanish], MFCD00057167, Dtic-Dome (TN), DTXCID20369, Imidazole-4-carboxamide, 5-(3,3-dimethyl-1-triazeno)-, DTXSID0020369, 4-[(1E)-3,3-Dimethyltriaz-1-en-1-yl]-1H-imidazole-5-carboxamide, 5-(3,3-Dimethyl-1-triazenyl)imidazole-4-carboxamide, 4-[(E)-dimethylaminodiazenyl]-1H-imidazole-5-carboxamide, NCGC00091861-01, Dacatic, DACARBAZINE (IARC), DACARBAZINE [IARC], dacarbazina, DACARBAZINE (MART.), DACARBAZINE [MART.], DACARBAZINE (USP-RS), DACARBAZINE [USP-RS], Dacarbazine-d6, 5- (3,3-Dimethyl-1-triazenyl) imidazole-4-carboxamide, 5-(dimethyltriaz-1-en-1-yl)-1H-imidazole-4-carboxamide, HSDB 3219, Dacarbazino, DACARBAZINE (EP MONOGRAPH), DACARBAZINE [EP MONOGRAPH], DACARBAZINE (USP MONOGRAPH), DACARBAZINE [USP MONOGRAPH], 750512-03-9, CAS-4342-03-4, Dimethyl Triazeno Imidazole Carboxamide, Carboxamide, Dimethyl Imidazole, Imidazole Carboxamide, Dimethyl, SR-05000001598, Dacarbazin, Dakarbazin, 5-(3,3-dimethyltriaz-1-en-1-yl)-1H-imidazole-4-carboxamide, Asercit, Detimedac, Fauldetic, CCRIS 190, Dacarbazine (JAN/USP/INN), dtic-aome, 5-(Dimethyltriazeno)imidazole-4-carboximide, Dacarbazine-DTIC, (E)-Dacarbazine, Dimethyltriazenoimidazolecarboxamide, Dacarbazina Almirall, EINECS 224-396-1, 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide, 5(or 4)-(dimethyltriazeno)imidazole-4(or 5)-carboxamide, 5-(3,3-Dimethyl-1-triazenyl)-1H-imidazole-4-carboxamide, (5E)-5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide, 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide, AI3-52825, DACARBAZINE [MI], 4-(or 5)-(3,3-Dimethyl-1-triazeno)imidazole-5(or 4)-carboxamide, DACARBAZINE [INN], DACARBAZINE [JAN], Dacarbazine (DTIC-Dome), DACARBAZINE [HSDB], DACARBAZINE [USAN], CHEMBL476, UNII-7GR28W0FJI, DACARBAZINE [VANDF], SCHEMBL5560, SCHEMBL5561, DACARBAZINE [WHO-DD], DACARBAZINE [WHO-IP], 5-[(1E)-dimethyltriaz-1-en-1-yl]-1H-imidazole-4-carboxamide, SPECTRUM1500218, SCHEMBL1014331, Carboxamide, 5-(3,3-dimethyl-1-triazeno)imidazole-4-, CHEBI:94587, HMS501A08, DACARBAZINE [ORANGE BOOK], Imidazole-4-carboxamide, 5-(3,3-dimethyl-1-triazenyl)-, L01AX04, CHEBI:177836, BDBM233149, HMS2090A20, HMS2091I20, Pharmakon1600-01500218, DACARBAZINUM [WHO-IP LATIN], HY-B0078, Tox21_111171, Tox21_201010, CCG-35381, CCG-36068, CCG-40272, Imidazole-4(or 5)-carboxamide, 5(or 4)-(3,3-dimethyl-1-triazeno)-, NSC759610, NSC799994, s1221, Imidazole Carboxamide Dimethyltriazeno, WLN: T5M CNJ DVZ ENUNN1&1, AKOS005220502, AKOS015850745, AKOS026750028, Dimethyl Triazeno Imidazol Carboxamide, dimethyl-triazeno-imidazole carboxamide, Dimethyl-triazeno-imidazole-carboximide, Tox21_111171_1, CS-1772, DB00851, KS-5186, NSC-759610, NSC-799994, Dacarbazine [USAN:USP:INN:BAN:JAN], Dimethyl (triazeno) imidazolecarboxamide, NCGC00091861-02, NCGC00091861-03, NCGC00091861-04, NCGC00091861-05, NCGC00091861-07, NCGC00188955-01, NCGC00258563-01, 94361-71-4, SBI-0051328.P003, WR-139007, FT-0603657, FT-0624397, FT-0665443, C06936, Carboxamide,3-dimethyl-1-triazeno)imidazole-4-, D00288, EN300-7357020, Imidazole-4-carboxamide,3-dimethyl-1-triazeno)-, A826278, SR-05000001598-1, SR-05000001598-3, W-106228, 1H-Imidazole-4-carboxamide,3-dimethyl-1-triazenyl)-, 4-[(E)-dimethylaminoazo]-1H-imidazole-5-carboxamide, BRD-K35520305-001-04-5, BRD-K35520305-001-07-8, (5E)-5-(dimethylaminohydrazono)imidazole-4-carboxamide, (5Z)-5-(dimethylaminohydrazono)imidazole-4-carboxamide, Imidazole-4(or 5)-carboxamide,3-dimethyl-1-triazeno)-, Z2289761610, 5-(3,3-Dimethyltriaz-1-enyl)-1H-imidazole-4-carboxamide, 5-(3-3-dimethyl-1-triazenyl)-1H-imidazole-4-carboxamide, Dacarbazine, British Pharmacopoeia (BP) Reference Standard, Dacarbazine, European Pharmacopoeia (EP) Reference Standard, (5E)-5-(dimethylaminohydrazinylidene)-4-imidazolecarboxamide, 4-(or 5)-(3,3-Dimethyl-1-triazeno)imidazole-5(or 4)-carboxamide, Dacarbazine, United States Pharmacopeia (USP) Reference Standard, IMIDAZOLE-4-CARBOXAMIDE, 5-(3,3-DIMETHYL-1-TRIAZENO1-, 1 H-IMIDAZOLE-4-CARBOXAMIDE, 5-(3,3-DIMETHYL-1-TRIAZENYL)-, Dacarbazine, Pharmaceutical Secondary Standard; Certified Reference Material