3-phenyl-2-propenal, 3-phenylprop-2-enaldehyde, beta-phenylacrolein, cinnamaldehyde, cinnamic aldehyde, cinnamic aldehyde, (E)-isomer, supercinnamaldehyde, trans-3-phenylprop-2-enaldehyde, trans-cinnamaldehyde



| Name | Cinnamaldehyde | ||
| PubChem CID | 637511 | ||
| Molecular Weight | 132.16g/mol | ||
| Synonyms |
3-phenyl-2-propenal, 3-phenylprop-2-enaldehyde, beta-phenylacrolein, cinnamaldehyde, cinnamic aldehyde, cinnamic aldehyde, (E)-isomer, supercinnamaldehyde, trans-3-phenylprop-2-enaldehyde, trans-cinnamaldehyde |
||
| Formula | C₉H₈O | ||
| SMILES | C1=CC=C(C=C1)C=CC=O | ||
| InChI | 1S/C9H8O/c10-8-4-7-9-5-2-1-3-6-9/h1-8H/b7-4+ | ||
| InChIKey | KJPRLNWUNMBNBZ-QPJJXVBHSA-N | ||
| CAS Number | 104-55-2 | ||
| ChEMBL ID | CHEMBL293492 | ||
| ChEBI ID | CHEBI:16731 | ||
| Herb ID | HBIN020653 | ||
| Drug Bank ID | DB14184 | ||
| KEGG ID | C00903 | ||
| Toxicity | Organism | Test Type | Route(Dose) |
| rat | LD50 | intraperitoneal(165 mg/kg) | |
| mouse | LD50 | intraperitoneal(254 mg/kg) | |
| rat | LD50 | oral(322 mg/kg) | |
| Structure | 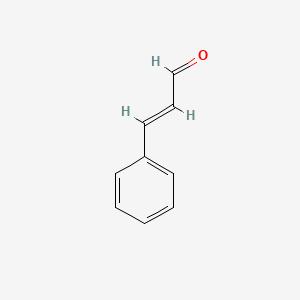
|
Download
2D
MOL
3D
MOL
|
|
| Chineses Pinyin | RouGui | ||
| Use Part | Bark | ||
| Flavor | Pungent; Sweet | ||
| Meridian Tropism | Kidney; Spleen; Heart; Liver | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Laurales
-->Family: Lauraceae
-->Genus: Cinnamomum
-->Species: Cinnamomum aromaticum
|
||
| Pair Name | Cinnamaldehyde, Dacarbazine | |||
| Partner Name | Dacarbazine | |||
| Disease Info | [ICD-11: 2C30] | Melanoma | Investigative | |
| Biological Phenomena | Inhibition-->Glycolysis | |||
| Gene Regulation | Down-regulation | Expression | ENO1 | hsa2023 |
| In Vitro Model | A-375 | Amelanotic melanoma | Homo sapiens (Human) | CVCL_0132 |
| In Vivo Model | A375 cells (1×10⁷) were injected into nude mice. When the tumors had formed for one week, the mice were treated with DITC (25, 50, or 100 mg/kg/day) and CA (15, 30, or 60 mg/kg/day). | |||
| Result | As a covalent inhibitor of ENO1, CA combined with DTIC may be beneficial in patients with drug resistance in antimelanoma therapy. | |||
