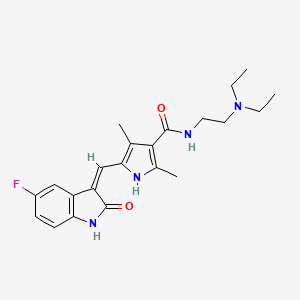
Sunitinib, 557795-19-4, Sutent, SU11248, SU-11248, sunitinibum, Su-011248, Sunitinib Base, 342641-94-5, (Z)-N-(2-(Diethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide, N-[2-(diethylamino)ethyl]-5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]methyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide, Sunitinib (INN), Sunitinib [INN], NSC-750690, SU 11248, UNII-V99T50803M, CHEBI:38940, HSDB 7932, N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide, CHEMBL535, N-(2-(Diethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide, NSC-736511, V99T50803M, SU011248, NSC750690, Sunitinib, Free base, NSC 736511, NSC 750690, 1H-Pyrrole-3-carboxamide, N-(2-(diethylamino)ethyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-, n-(2-(diethylamino)ethyl)-5-((z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl)-2,4-dimethyl-1h-pyrrole-3-carboxamide, N-(2-(DIETHYLAMINO)ETHYL)-5-((Z)-(5-FLUORO-2-OXO-1,2-DIHYDRO-3H-INDOL-3-YLIDENE)METHYL)-2,4-DIMETHYL-1H-PYRROLE-3-CARBOXAMIDE, Sunitinib (free base), Sunitinib [INN:BAN], NCGC00164631-01, MFCD09260778, C22H27FN4O2, (Z)-Sunitinib, 1H-Pyrrole-3-carboxamide, N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-, 5-(5-fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylamino-ethyl)-amide, 5-[5-fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylamino-ethyl)-amide, Sutent (free base), KS-5022, SUNITINIB [MI], 1126641-10-8, SUNITINIB [VANDF], SCHEMBL8081, SUNITINIB [WHO-DD], SUNITINIB [EMA EPAR], BDBM4814, GTPL5713, Sutent, Sunitinib, SU11248, DTXCID9021134, CHEBI:91430, EX-A553, L01XE04, WINHZLLDWRZWRT-ATVHPVEESA-N, BCPP000057, K00588a, (E)-N-[2-(Diethylamono)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide, N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide, HY-10255A, NSC800937, s7781, AKOS015908193, AKOS025312424, CCG-268638, CS-1670, DB01268, NSC-800937, NCGC00164631-02, NCGC00164631-04, 5-(5-fluoro-2-oxo-1,2-dihydroindolylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, BD164426, 1,2,4,5-tetramethylpyrrole-3-carboxamide, AM20090630, FT-0651493, FT-0659515, NS00004661, D08552, EN300-323230, AB01273976-01, AB01273976-02, AB01273976_04, A822143, A830806, Q417542, SR-00000000005, SR-00000000005-2, Q27163278, Z2568722545, 1H-Pyrrole-3-carboxamide,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-, 5-[5-fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid(2-diethylamino-ethyl)-amide, N-(2-(Diethylamino)ethyl)-5-((Z)-(5-fluoro-1 pound not2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2 pound not4-dimethyl-1H-pyrrole-3-carboxamide, N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide, N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide, N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carbo