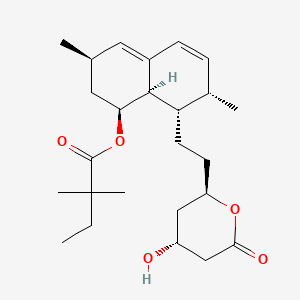
simvastatin, 79902-63-9, Zocor, Synvinolin, Sinvacor, Denan, Lipex, MK-733, Sivastin, Lodales, Cholestat, Simvastatine, Colemin, Medipo, Pantok, Simovil, Labistatin, Simvastatina, Simvastatinum, Velostatin, Coledis, Corolin, Nivelipol, Rendapid, Vasotenal, Zorced, Rechol, Zocord, Simvastatin (Zocor), Simvastatin lactone, Lipovas, Simcard, Simvacor, Simvoget, Simlup, Zosta, MK-0733, Simvastatinum [Latin], (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate, DRG-0320, CCRIS 7558, MK 0733, HSDB 7208, UNII-AGG2FN16EV, AGG2FN16EV, 2,2-Dimethylbutyric acid, 8-ester with (4R,6R)-6-(2-((1S,2S,6R,8S,8aR)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2H-pyran-2-one, Statin, NSC-758706, L 644128-000U, BRN 4768037, CHEBI:9150, DTXSID0023581, C10AA01, DTXCID103581, (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate, Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester, SIMCOR COMPONENT SIMVASTATIN, Simvastatin [USAN:USP:INN:BAN], VYTORIN COMPONENT SIMVASTATIN, MFCD00072007, NSC633782, NSC 758706, [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate, Simvastatinum (Latin), Simvastatine [French], Simvastatina [Spanish], SIMVASTATIN (MART.), SIMVASTATIN [MART.], SIMVASTATIN (USP-RS), SIMVASTATIN [USP-RS], Butanoic acid, 2,2-dimethyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester, Butanoic acid, 2,2-dimethyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester, (1S-(1alpha,3alpha,7beta,8beta(2S*,4S*),8abeta))-, SIMVASTATIN (EP MONOGRAPH), SIMVASTATIN [EP MONOGRAPH], SIMVASTATIN (USP MONOGRAPH), SIMVASTATIN [USP MONOGRAPH], Simvastatin (USAN:USP:INN:BAN), Simvast CR, (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate, [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxo-tetrahydropyran-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate, SMR000718785, Zocor (TN), Simvastatin & Primycin, MK733, SR-05000001894, MK 733, Kolestevan, Lipinorm, Modutrol, Simvotin, Sinvascor, Valemia, Eucor, Nor-Vastina, Simvastatin,(S), simvastatin predrug, (+)-Simvastatin, NCGC00016940-01, inactive simvastatin, 2,2-Dimethylbutyric acid, 8-ester with (4R,6R)-6-[2-[(1S,2S,6R,8S,8aR)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl]ethyl]tetrahydro-4-hydroxy-2H-pyran-2-one, TNP00259, Prestwick_171, Simvastatin- Bio-X, CAS-79902-63-9, FLOLIPID, KS-1113, Spectrum_001717, SpecPlus_000895, SIMVASTATIN [MI], Prestwick0_000865, Prestwick1_000865, Prestwick2_000865, Prestwick3_000865, Spectrum2_001671, Spectrum3_000669, Spectrum4_000632, Spectrum5_001428, SIMVASTATIN [INN], SIMVASTATIN [JAN], SIMVASTATIN [HSDB], SIMVASTATIN [USAN], SIMVASTATIN [VANDF], SCHEMBL2471, CHEMBL1064, BSPBio_000909, BSPBio_002337, KBioGR_001244, KBioSS_002197, SIMVASTATIN [WHO-DD], MLS001304029, MLS001333077, MLS001333078, MLS002154038, MLS006011866, BIDD:GT0769, DivK1c_006991, SPECTRUM1504236, SPBio_001881, SPBio_002830, BPBio1_001001, GTPL2955, Simvastatin (JP17/USP/INN), Simvastatin, analytical standard, BCBcMAP01_000007, KBio1_001935, KBio2_002197, KBio2_004765, KBio2_007333, KBio3_001557, RYMZZMVNJRMUDD-HGQWONQESA-, SIMVASTATIN [ORANGE BOOK], HMS1570N11, HMS1922H13, HMS2089D12, HMS2093E06, HMS2097N11, HMS2231N22, HMS3259B12, HMS3412P08, HMS3676P08, HMS3714N11, HMS3884G10, Pharmakon1600-01504236, BUTANOIC ACID, 2,2-DIMETHYL-, 1,2,3,7,8,8A-HEXAHYDRO-3,7-DIMETHYL-8-(2-(TETRAHYDRO-4-HYDROXY-6-OXO-2H-PYRAN-2-YL)ETHYL)-1-NAPHTHALENYL ESTER, (1S-(1.ALPHA.,3.ALPHA.,7.BETA.,8.BETA.(2S*,4S*),8A.BETA.))-, Tox21_110696, Tox21_300400, BBL024390, BDBM50139181, CCG-39069, NSC758706, s1792, STK801938, AKOS005111006, AKOS015842733, SIMVASTATIN COMPONENT OF SIMCOR, Simvastatin, >=97% (HPLC), solid, Tox21_110696_1, AC-1530, DB00641, NC00719, NSC-633782, SIMVASTATIN COMPONENT OF VYTORIN, MRF-0000729, NCGC00017324-01, NCGC00017324-02, NCGC00017324-03, NCGC00017324-04, NCGC00017324-05, NCGC00017324-07, NCGC00017324-08, NCGC00017324-09, NCGC00254418-01, 2,2-Dimethylbutanoic acid (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester, BS164407, HY-17502, SBI-0206773.P001, Simvastatin 100 microg/mL in Acetonitrile, NS00008782, S0509, EN300-52503, D00434, AB00053395-07, AB00053395-08, AB00053395-10, AB00053395_11, AB00053395_13, A839783, Q670131, SR-05000001894-1, SR-05000001894-2, BRD-K22134346-001-05-8, BRD-K22134346-001-11-6, BRD-K22134346-001-15-7, Z754918914, Simvastatin, British Pharmacopoeia (BP) Reference Standard, Simvastatin, European Pharmacopoeia (EP) Reference Standard, Simvastatin, United States Pharmacopeia (USP) Reference Standard, Simvastatin, Pharmaceutical Secondary Standard; Certified Reference Material, Simvastatin for peak identification, European Pharmacopoeia (EP) Reference Standard, (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate, (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbu, (1S,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate, (1S-(1alpha,3alpha,7beta,8beta(2S*,4S*),8abeta))-1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl 2,2-dimethylbutanoate, InChI=1/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1