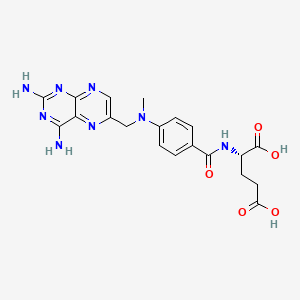
methotrexate, 59-05-2, Rheumatrex, Amethopterin, Methylaminopterin, Metatrexan, Hdmtx, Abitrexate, Trexall, Mexate, Ledertrexate, Methylaminopterinum, (S)-2-(4-(((2,4-Diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)pentanedioic acid, Methotrexatum, Antifolan, MTX, 4-Amino-10-methylfolic acid, Amethopterine, Metotrexato, Emtexate, Maxtrex, Rasuvo, Methotrexate hydrate, A-Methopterin, L-Amethopterin, A-Methpterin, Amethopterin L-, Folex-Pfs, Methotrexat-Ebewe, NSC-740, Methotrexate, L-, N-Bismethylpteroylglutamic acid, Farmitrexat, Medsatrexate, Methoblastin, Methotextrate, Methotrexat, Metotressato, Brimexate, Emthexat, Emthexate, Fauldexato, Lantarel, Lumexon, Metrotex, Novatrex, Otrexup, Tremetex, Trexeron, Trixilem, Texate, Metex, Mexate-Aq, alpha-Methopterin, CL-14377, Folex, NCI-C04671, JYLAMVO, Methotrexate Lpf, CCRIS 1109, Nordimet, Xatmep, EMT 25,299, 133073-73-1, 4-Aminomethylpteroylglutamic acid, HSDB 3123, NSC 740, CL 14377, UNII-YL5FZ2Y5U1, Methotrexatum [INN-Latin], Metotrexato [INN-Spanish], EINECS 200-413-8, YL5FZ2Y5U1, R 9985, REDITREX, NSC740, DTXSID4020822, CHEBI:44185, AI3-25299, TCMDC-125858, X 133, 4-Amino-N(sup 10)-methylpteroylglutamic acid, ADX-2191, MPI-2505, WR-19039, CHEMBL34259, DTXCID80822, (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid, R-9985, Kyselina 4-amino-N(sup 10)-methylpteroylglutamova, N-(p-(((2,4-Diamino-6-pteridyl)methyl)methylamino)benzoyl)glutamic acid, L-(+)-N-(p-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)glutamic acid, N-(4-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)-L-glutamicacid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-L-glutamic acid, N-(p-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)-L-(+)-glutamic acid, N-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}phenyl)carbonyl]-L-glutamic acid, Methotrexate [USAN:USP:INN:BAN:JAN], NCGC00025060-04, 4-amino-N(10)-methylpteroylglutamic acid, MFCD00150847, Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)methylamino)benzoyl)-L-glutamova, METHOTREXATE (IARC), METHOTREXATE [IARC], Methotrexatum (INN-Latin), Metotrexato (INN-Spanish), (2S)-2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}phenyl)formamido]pentanedioic acid, Glutamic acid, N-(p-(((2,4-diamino-6-pteridinyl)methyl)methylamino)benzoyl)-, L-, METHOTREXATE (MART.), METHOTREXATE [MART.], METHOTREXATE (USP-RS), METHOTREXATE [USP-RS], Metotressato [DCIT], Methotrexate, d-, METHOTREXATE (EP MONOGRAPH), METHOTREXATE (USP IMPURITY), METHOTREXATE [EP MONOGRAPH], METHOTREXATE [USP IMPURITY], METHOTREXATE (USP MONOGRAPH), METHOTREXATE [USP MONOGRAPH], MLS001401431, [3H]methotrexate, Methotrexate (hydrate), Methotrexate (USAN:USP:INN:BAN:JAN), SMR000112001, [3H]-methotrexate, folic acid antagonist, 4-Amino-10-methylfolic acid hydrate, SR-01000075682, SMR000449324, Methotrexate (1.0mg/mL in Methanol with 0.1N NaOH), TCMDC-125488, Metolate, Antifolan hydrate, Intradose-MTX, MTX hydrate, 1dhi, 1dhj, 2drc, 4ocx, (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]pentanedioic acid, CAS-59-05-2, Prestwick_322, Otrexup (TN), Xatmep (TN), L-METHOTREXATE, OTREXUP PFS, Kyselina 4-amino-N(sup 10)-methylpteroylglutamova [Czech], Methylaminopterin; MTX, DL-Amethopterin hydrate, Spectrum_001836, Tocris-1230, 4kn0, Methylaminopterin hydrate, Prestwick0_000135, Prestwick1_000135, Prestwick2_000135, Spectrum2_001077, Spectrum3_000497, Spectrum4_000616, Spectrum5_000958, Abitrexate (Methotrexate), METHOTREXATE [MI], METHOTREXATE [INN], METHOTREXATE [JAN], METHOTREXATE [HSDB], METHOTREXATE [USAN], NCIMech_000767, SCHEMBL3711, Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)methylamino)benzoyl)-L-glutamova [Czech], METHOTREXATE [VANDF], BIDD:PXR0175, Lopac0_000020, KBioGR_001172, KBioSS_002341, Methotrexate Methylaminopterin, MLS000049968, MLS002154208, DivK1c_000114, METHOTREXATE [WHO-DD], METHOTREXATE [WHO-IP], SPBio_001094, SPBio_002149, Amethopterin (hydrate); CL14377 (hydrate); WR19039 (hydrate), AMY235, cid_126941, cid_165528, GTPL4674, GTPL4815, SCHEMBL12421860, SCHEMBL23111732, BDBM18050, BDBM66082, HMS500F16, KBio1_000114, KBio2_002338, KBio2_004906, KBio2_007474, KBio3_001493, Methotrexate (JP17/USP/INN), L01BA01, L04AX03, g301, NINDS_000114, Bio1_000486, Bio1_000975, Bio1_001464, HMS1568K12, HMS2095K12, HMS2233O18, HMS3260C21, HMS3414L09, HMS3678L07, HMS3712K12, METHOTREXATE [ORANGE BOOK], APC-2002, BCP13701, GLUTAMIC ACID, N-(P-(((2,4-DIAMINO-6-PTERIDINYL)METHYL)METHYLAMINO)BENZOYL)-, L-(+)-, MPI-5004, Tox21_110944, Tox21_300269, Tox21_500020, CCG-35800, EMT-25299, METHOTREXATUM [WHO-IP LATIN], MFCD00064370, s1210, STL535338, (4-(((2,4-diaminopteridin-6-yl)methyl)(methyl)amino)benzoyl)-L-glutamic acid, AKOS016340329, Tox21_110944_1, CL14377, CS-1732, DB00563, KS-5093, LP00020, SDCCGSBI-0050009.P003, IDI1_000114, SMP2_000020, (methyl)amino)benzamido)pentanedioic acid, NCGC00025060-01, NCGC00025060-02, NCGC00025060-03, NCGC00025060-05, NCGC00025060-06, NCGC00025060-07, NCGC00025060-08, NCGC00025060-09, NCGC00025060-10, NCGC00025060-11, NCGC00025060-12, NCGC00025060-13, NCGC00025060-15, NCGC00025060-16, NCGC00254216-01, NCGC00260705-01, HY-14519, EU-0100020, FT-0601523, FT-0630651, G-301, NS00098764, SW198601-3, Methotrexate 1.0 mg/ml in Dimethyl Sulfoxide, C01937, D00142, EN300-119523, DL-4-Amino-N10-methylpteroylglutamic acid hydrate, Q422232, SR-01000597411, W-60383, (S)-2-(4-(((2,4-diaminopteridin-6-yl)methyl), Q-201366, SR-01000075682-1, SR-01000075682-2, SR-01000075682-6, SR-01000597411-1, W-105347, BRD-K59456551-001-09-3, BRD-K59456551-001-11-9, WLN: T66 BN DN GN JNJ CZ EZ H1N1&R DVMYVQ2VQ, Z1521553982, 4-AMINO-4-DEOXY-N(SUP 10)-METHYLPTEROYLGLUTAMATE, Methotrexate, European Pharmacopoeia (EP) Reference Standard, Methotrexate, United States Pharmacopeia (USP) Reference Standard, Glutamic acid,4-diamino-6-pteridinyl)methyl] methylamino]benzoyl]-, L-(+)-, L-Glutamic acid,4-diamino-6-pteridinyl)methyl]- methylamino]benzoyl]-, (S)-2-(4-(((2,4-Diaminopteridin-6-yl)methyl)-(methyl)amino)benzamido)pentanedioic acid, GLUTAMIC ACID, N-(P-(((2,4-DIAMINO-6-PTERIDINYL)METHYL)METHYLAMINO)BENZOYL)-,L, L-Glutamic acid,N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-,hydrate(9ci), Methotrexate for peak identification, European Pharmacopoeia (EP) Reference Standard, Methotrexate for system suitability, European Pharmacopoeia (EP) Reference Standard, Methotrexate, Pharmaceutical Secondary Standard; Certified Reference Material, N-(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}benzoyl)-L-glutamic acid, N-[4-[[(2,4-Diamino-6-pteridinyl)methyl] methylamino]benzoyl]-L-glutamic acid, (2S)-2-((4-(((2,4-DIAMINOPTERIDIN-6-YL)METHYL)(METHYL)AMINO)BENZOYL)AMINO)PENTANEDIOIC ACID, (2S)-2-[[[4-[(2,4-diamino-6-pteridinyl)methyl-methylamino]phenyl]-oxomethyl]amino]pentanedioic acid;hydrate, (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]glutaric acid;hydrate, (2S)-2-[[4-[[2,4-bis(azanyl)pteridin-6-yl]methyl-methyl-amino]phenyl]carbonylamino]pentanedioic acid;hydrate, 102613-64-9