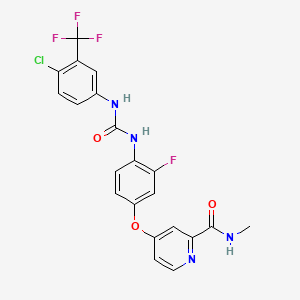
Regorafenib, 755037-03-7, BAY 73-4506, Stivarga, 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide, Regorafenibum, BAY73-4506, Regorafenib (BAY 73-4506), BAY-73-4506, 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide, UNII-24T2A1DOYB, REGORAFENIB ANHYDROUS, 24T2A1DOYB, CHEBI:68647, Bay-734506, 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide, CHEMBL1946170, DTXSID60226441, 4-(4-(((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl)amino)-3-fluorophenoxy)-n-methylpyridine-2-carboxamide, Regorafenib-13C-d3, Stivarga (TN), 2-Pyridinecarboxamide,4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-, UNII-MGN125FS9D, REGORAFENIB (USP-RS), CHEBI:68646, DTXSID70144349, REGORAFENIB (USP MONOGRAPH), Regorafenib [INN], 835621-08-4, Regorafenib [USAN:INN], C21H15ClF4N4O3, 4-(4-(((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl}amino)-3-fluorophenoxy)-N-methylpyridine-2-carboxamide, REGORAFENIB [MI], Regorafenib (USAN/INN), REGORAFENIB [VANDF], BAY73-4506 hydrochloride, REGORAFENIB [WHO-DD], MLS006010303, Regorafenib crystalline form I, SCHEMBL432230, Regorafenib,BAY 73-4506, GTPL5891, DTXCID8066840, DTXCID60148932, EX-A058, L01XE21, BCPP000352, HMS3654K16, HMS3672E15, 755037-03-7 , Regorafenib, BCP02105, BKD17855, BDBM50363397, MFCD16038047, NSC763932, NSC800865, s1178, AKOS015951107, AM81251, BCP9000384, CCG-269571, CS-0170, DB08896, NSC-763932, NSC-800865, SB16819, NCGC00263138-01, NCGC00263138-13, NCGC00263138-19, 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluoro-phenoxy]-N-methyl-pyridine-2-carboxamide, AC-25075, AC-31116, AS-16304, HY-10331, SMR004701370, FT-0674338, NS00040710, R0142, SW218097-2, BAY 73-4506 , Fluoro-Sorafenib, cas:835621-07-3;Regorafenib hydrochloride, Regorafenib (BAY73-4506,Fluoro-Sorafenib), A25020, D10138, Regorafenib (BAY73-4506,Fluoro-Sorafenib)?, AB01565826_02, SR-01000941571, Q3891664, SR-01000941571-1, BRD-K16730910-001-02-4, Z2235801830, 755037-03-7 (anhydrous); 1019206-88-2 (hydrate), 2-Pyridinecarboxamide, 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-, 4-[4-({[4-Chloro-3-(trifluoromethy)phenyl]carbamoyl}amino)-3-fluorophenoxy]-1-methylpyridine-2-carboxamide, 4{4-[3-(4-chloro-3-trifluoromethylphenyl)-ureido]-3-fluorophenoxy}-pyridine-2-carboxylic acid methylamide, Regorafenib;1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(2-fluoro-4-(2-(methylcarbamoyl)pyridin-4-yloxy)phenyl)urea