


| Name | Daurinoline | ||
| PubChem CID | 12309092 | ||
| Molecular Weight | 610.7g/mol | ||
| Formula | C₃₇H₄₂N₂O₆ | ||
| SMILES | CN1CCC2=CC(=C(C=C2C1CC3=CC=C(C=C3)OC4=C(C=CC(=C4)CC5C6=CC(=C(C=C6CCN5C)OC)OC)O)OC)O | ||
| InChI | 1S/C37H42N2O6/c1-38-14-12-25-19-33(41)34(42-3)21-28(25)30(38)16-23-6-9-27(10-7-23)45-35-18-24(8-11-32(35)40)17-31-29-22-37(44-5)36(43-4)20-26(29)13-15-39(31)2/h6-11,18-22,30-31,40-41H,12-17H2,1-5H3/t30-,31-/m1/s1 | ||
| InChIKey | APIHNXDZCYDPTF-FIRIVFDPSA-N | ||
| Herb ID | HBIN022789 | ||
| Structure | 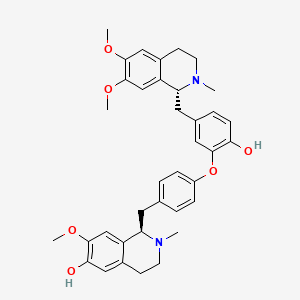
|
Download
2D
MOL
3D
MOL
|
|
| Chineses Pinyin | ShanDouGen | ||
| Use Part | Root, Rhizome | ||
| Habitat | GuangXi | ||
| Flavor | Bitter | ||
| Meridian Tropism | Lung, Stomach | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Fabales
-->Family: Fabaceae
-->Genus: Sophora
-->Species: Sophora tonkinensis
|
||
| Chineses Pinyin | BeiDou | ||
| Habitat | HeiLongJiang, JiLin, LiaoNing, BeiJing, TianJin, HeBei, ShanXi, NeiMengGu, JiangSu, ZheJiang, AnHui, FuJian, JiangXi, ShanDong, ShangHai, Shaanxi, NingXia, GanSu, ShanDong | ||
| Flavor | Bitter | ||
| Meridian Tropism | Lung, Stomach, Large intestine | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Ranunculales
-->Family: Menispermaceae
-->Genus: Menispermum
-->Species: Menispermum dauricum
|
||
| Chineses Pinyin | BianFuGe | ||
| Use Part | Rattan | ||
| Habitat | HeiLongJiang, JiLin, LiaoNing, BeiJing, TianJin, HeBei, ShanXi, NeiMengGu, JiangSu, ZheJiang, AnHui, FuJian, JiangXi, ShanDong, ShangHai, ShanXi, NingXia, GanSu, ShanDong | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Ranunculales
-->Family: Menispermaceae
-->Genus: Menispermum
-->Species: Menispermum dauricum
|
||
| Pair Name | Daurinoline, Sorafenib | |||
| Partner Name | Sorafenib | |||
| Disease Info | [ICD-11: 2C12] | Hepatocellular carcinoma | Investigative | |
| Biological Phenomena | Inhibition-->Vasculogenic mimicry | |||
| Gene Regulation | Down-regulation | Activity | RHOA | hsa387 |
| Down-regulation | Activity | ROCK2 | hsa9475 | |
| Down-regulation | Phosphorylation | AKT1 | hsa207 | |
| Down-regulation | Phosphorylation | MAPK1 | hsa5594 | |
| Down-regulation | Phosphorylation | MAPK3 | hsa5595 | |
| Down-regulation | Expression | KDR | hsa3791 | |
| Down-regulation | Expression | CDK2 | hsa1017 | |
| Down-regulation | Expression | CDK4 | hsa1019 | |
| Down-regulation | Expression | CCNE1 | hsa898 | |
| Down-regulation | Expression | CCND1 | hsa595 | |
| Up-regulation | Expression | CDKN1A | hsa1026 | |
| Up-regulation | Expression | PSMD9 | hsa5715 | |
| Down-regulation | Expression | BCL2 | hsa596 | |
| Up-regulation | Expression | BAX | hsa581 | |
| Up-regulation | Cleavage | CASP3 | hsa836 | |
| Up-regulation | Cleavage | CASP9 | hsa842 | |
| Up-regulation | Cleavage | PARP1 | hsa142 | |
| In Vitro Model | QGY-7703 | Human papillomavirus-related cervical adenocarcinoma | Homo sapiens (Human) | CVCL_6715 |
| MHCC97-H | Adult hepatocellular carcinoma | Homo sapiens (Human) | CVCL_4972 | |
| In Vivo Model | MHCC-97H cells were suspended in cold PBS at a density of 1×10⁷ cells/ml, and 100 μl of the cell suspension was subcutaneously injected into the right flank of each mouse. | |||
| Result | Our study provides insights into the molecular mechanisms underlying DS-induced inhibition of VM, which may facilitate the development of a novel clinical anti-HCC drug. Moreover, our findings suggest that the combination of DS and sorafenib constitutes a potential therapeutic strategy for HCC. | |||
