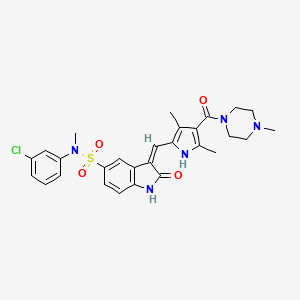
658084-23-2, SU11274, Met Kinase Inhibitor, SU 11274, PKI-SU11274, SU-11274, (Z)-N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide, SU-MI-2, CHEMBL261641, (3Z)-N-(3-Chlorophenyl)-3-({3,5-dimethyl-4-[(4-methylpiperazin-1-yl)carbonyl]-1H-pyrrol-2-yl}methylene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide, MFCD08276928, (3Z)-N-(3-Chlorophenyl)-3-((3,5-dimethyl-4-((4-methylpiperazin-1-yl)carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide, (3Z)-N-(3-Chlorophenyl)-3-[[3,5-dimethyl-4-[(4-methyl-1-piperazinyl)carbonyl]-1H-pyrrol-2-yl]methylene]-2,3-dihydro-N-methyl-2-oxo-1H-indole-5-sulfonamide, (3Z)-N-(3-chlorophenyl)-3-[[3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl]methylidene]-N-methyl-2-oxo-1H-indole-5-sulfonamide, (3z)-N-(3-Chlorophenyl)-3-({3,5-Dimethyl-4-[(4-Methylpiperazin-1-Yl)carbonyl]-1h-Pyrrol-2-Yl}methylene)-N-Methyl-2-Oxoindoline-5-Sulfonamide, (3Z)-N-(3-chlorophenyl)-3-({3,5-dimethyl-4-[(4-methylpiperazin-1-yl)carbonyl]-1H-pyrrol-2-yl}methylidene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide, Met Kinase, SCHEMBL93711, SCHEMBL93713, MLS006010961, GTPL5057, DTXSID20429552, EX-A183, CHEBI:190974, FPYJSJDOHRDAMT-KQWNVCNZSA-N, BCPP000061, HMS3229G21, K00593a, BDBM50341636, NSC747693, s1080, AKOS015994564, CCG-206768, ES-0032, EX-5962, NSC-747693, NCGC00165902-01, NCGC00165902-04, (3Z)-N-(3-chlorophenyl)-3-[[3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl]methylene]-N-methyl-2-oxo-indoline-5-sulfonamide, (Z)-N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(1-methylpiperazine-4-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide, AC-28396, SMR004702765, SU-011274, SU11274 (PKI-SU11274), FT-0700347, SU 11274, >=98% (HPLC), powder, SU11274 (PKI-SU11274)?, A15738, Met Kinase Inhibitor - CAS 658084-23-2, J-522999, BRD-K02965346-001-01-8, BRD-K02965346-001-07-5, Q27088888, (3Z)-N-(3-chlorophenyl)-3-[[3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl]methylidene]-N-methyl-2-oxo-1H-indole-5-sulonamide, 1h-indole-5-sulfonamide, n-(3-chlorophenyl)-3-[[3,5-dimethyl-4-[(4-methyl-1-piperazinyl)carbonyl]-1h-pyrrol-2-yl]methylene]-2,3-dihydro-n-methyl-2-oxo-, (3z)-, N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(1-methylpiperazine-4-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide, N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide, N-(3-Chlorophenyl)-n-methyl-3-[[3,5-dmethyl-4-[(4-methylpperazn-1-yl)carbonyl]-1h-pyrrol-2-yl]methylene]-2-oxo-2,3-dhydro-1h-ndole-5 -sulfonamde