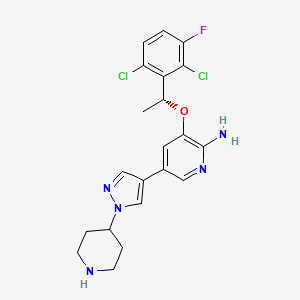
Crizotinib, 877399-52-5, PF-02341066, (R)-crizotinib, PF-2341066, Xalkori, PF 2341066, (R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine, Crizotinib (PF-02341066), 3-[(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-2-pyridinamine, crizotinibum, Crizotinib (PF-2341066), UNII-53AH36668S, CHEBI:64310, PF2341066, 53AH36668S, NSC-756645, CHEMBL601719, 3-[(1R)-1-(2,6-DICHLORO-3-FLUOROPHENYL)ETHOXY]-5-[1-(4-PIPERIDINYL)-1H-PYRAZOL-4-YL]PYRIDIN-2-AMINE, DTXSID701009329, 877399-52-5 (free base), NSC 756645, 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine, CRIZOTINIB (MART.), CRIZOTINIB [MART.], 2-Pyridinamine, 3-((1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(4-piperidinyl)-1H-pyrazol-4-yl)-, 3-[(1r)-1-(2,6-Dichloro-3-Fluorophenyl)ethoxy]-5-(1-Piperidin-4-Yl-1h-Pyrazol-4-Yl)pyridin-2-Amine, PF 02341066, 2-PYRIDINAMINE, 3-[(1R)-1-(2,6-DICHLORO-3-FLUOROPHENYL)ETHOXY]-5-[1-(4-PIPERIDINYL)-1H-PYRAZOL-4-YL]-, Xalkori (TN), Crizotinib [USAN], Crizotinib [USAN:INN], 3-((1R)-1-(2,6-DICHLORO-3-FLUOROPHENYL)ETHOXY)-5-(1-(PIPERIDIN-4-YL)-1H-PYRAZOL-4-YL)PYRIDIN-2-AMINE, VGH, Crizotinib- Bio-X, CRIZOTINIB [MI], CRIZOTINIB [INN], CRIZOTINIB [JAN], CRIZOTINIB [VANDF], CRIZOTINIB [WHO-DD], SCHEMBL93829, PF-2341066,Crizotinib, Crizotinib (JAN/USAN/INN), GTPL4903, CRIZOTINIB [ORANGE BOOK], Crizotinib, >=98% (HPLC), EX-A096, L01XE16, KTEIFNKAUNYNJU-GFCCVEGCSA-N, BCPP000116, DTXCID601436157, GLXC-04599, AMY10313, PF 2341066;(R)-3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine, BDBM50306682, MFCD12407409, NSC749005, NSC749769, NSC800080, AKOS015901233, AKOS015995207, CCG-264803, DB08865, GS-6178, NSC-749005, NSC-749769, NSC-800080, NCGC00250400-01, NCGC00250400-02, NCGC00250400-09, NCGC00250400-12, BC164334, HY-50878, NS00072165, SW202555-3, MET Tyrosine Kinase Inhibitor PF-02341066, D09731, J-510370, Q5186964, BRD-K78431006-001-01-1, BRD-K78431006-001-03-7, 877399-52-5, 877399-53-6 (acetate), Z2065417924, 3-(2,6-dichloro-3-fluorobenzyloxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine, (R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-am ine, (R)-3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine, 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-(1-piperidin-4-ylpyrazol-4-yl)pyridin-2-amine, 3-[(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyra zol-4-yl]-2-pyridinamine, 3-[(R)-1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine