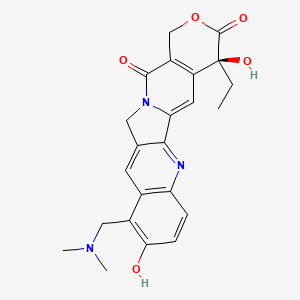
topotecan, 123948-87-8, Topotecan lactone, Hycamptamine, Hycamptin, (S)-Topotecan, Topotecane, Topotecanum, Nogitecan, Topotecane [INN-French], Topotecanum [INN-Latin], Topophore C, TopoCED, 9-Dimethylaminomethyl-10-hydroxycamptothecin, Topotecan Actavis, UNII-7M7YKX2N15, NSC-641007, 7M7YKX2N15, CCRIS 8163, CHEBI:63632, HSDB 8213, CHEMBL84, NSC609699, Hycamtin (TN), 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-, (4S)-, SK-S-104864-A, SKF-104864, (S)-10-((Dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, Hycamtamine, DTXSID3042685, FF-10850 (liposomal topotecan), NSC641007, (4S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, (S)-10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1,12-dihydro-14H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H)-dione, (S)-10-[(DIMETHYLAMINO)METHYL]-4-ETHYL-4,9-DIHYDROXY-1H-PYRANO[3',4':6,7]INOLIZINO[1,2-B]-QUINOLINE-3,14(4H,12H)-DIONE, 9-[(dimethylamino)methyl]-10-hydroxy-(4S)-camptothecin, SK&F-104864, Topotecane (INN-French), Topotecanum (INN-Latin), Topotecan [INN:BAN], (19S)-8-[(dimethylamino)methyl]-19-ethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione, 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, 10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-, (S)-, SKF-104864-A, SK&F-104864-A, 10-HYDROXY-9-((DIMETHYLAMINO)METHYL)-(20S)-CAMPTOTHECIN, CHEMBL1607, Topotecan (BAN), (19S)-8-[(dimethylamino)methyl]-19-ethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaene-14,18-dione, (S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]-quinoline-3,14(4H,12H)-dione, (S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 1H-PYRANO(3',4':6,7)INDOLIZINO(1,2-B)QUINOLINE-3,14(4H,12H)-DIONE, 10-((DIMETHYLAMINO)METHYL)-4-ETHYL-4,9-DIHYDROXY-, (4S)-, SK&F-S-104864A, SR-01000763672, SK&F 104864 A, 9-((dimethylamino)methyl)-10-hydroxy-(4S)-camptothecin, MFCD00870670, (4S)-10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, (s)-10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1H-pyrano(3',4':6,7)indolizino(1,2-b)-quinoline-3,14(4H,12H)-dione, 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-, (S)-, SKF-S 104864, Topotecanum (Latin), SKF 104864, TOPOTECAN [INN], TOPOTECAN [MI], 9 Dimethylaminomethyl 10 hydroxycamptothecin, TOPOTECAN [VANDF], SCHEMBL3836, TOPOTECAN [WHO-DD], NCIStruc1_001659, NCIStruc2_001796, BSPBio_002348, TOPOTECAN [EMA EPAR], cid_60699, GTPL7101, DTXCID1022685, EX-A834, HMS2090B20, HMS3715L03, BDBM50008935, BDBM50034026, HSCI1_000228, s9321, AKOS015966792, CCG-221171, DB01030, SMP2_000312, SMP2_000327, NCGC00014925-02, NCGC00014925-03, NCGC00014925-04, NCGC00014925-07, NCGC00014925-10, NCGC00014925-24, NCGC00178695-01, AC-11592, AC-34812, AS-75098, BP-29353, HY-13768, NCI60_004771, dimethylaminomethyl-ethyl-dihydroxy-[?]dione, NS00003193, dihydroxy(oxo)silane; dioxido(dioxo)molybdenum, D08618, EN300-117268, AB00641837-09, AB00641837-11, AB00641837-12, AB00641837-13, AB00641837-14, AB00641837_15, AB00641837_16, A805171, A892572, Q419953, SR-01000763672-3, SR-01000763672-4, BRD-K55696337-001-03-2, BRD-K55696337-003-08-7, Z1501485359, 9-((dimethylamino)methyl)-10-hydroxy-(20S)-camptothecin, Topotecan hydrochloride hydrate, >=98% (HPLC and enzymatic), (19S)-8-[(dimethylamino)methyl]-19-ethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.0(2),(1)(1).0?,?.0(1)?,(2)?]henicosa-1(21),2,4,6,8,10,15(20)-heptaene-14,18-dione, (20S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione, (4-Ethyl-4,9-dihydroxy-3,13-dioxo-3,4,12,13-tetrahydro-1H-2-oxa-6,12a-diaza-dibenzo[b,h]fluoren-10-ylmethyl)-dimethyl-ammonium, (S)-10-[(DIMETHYLAMINO)METHYL]-4-ETHYL-4,9-DIHYDROXY-1H-PYRANO[3'''',4'''':6,7]INOLIZINO[1,2-B]-QUINOLINE-3,14(4H,12H)-DIONE, (S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione monohydrochloride; Nogitecan hydrochloride; Hycamtin, (S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione, (S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione; hydrochloride, (S)-11-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione, (S)-11-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione; hydrochloride, 10-dimethylaminomethyl-4-ethyl-4,9-dihydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3'''',4'''':6,7]indolizino[1,2-b]quinoline-3,14-dione, 10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione, 10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (Topotecan), 10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione(Topotecan), 10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione; hydrochloride, 1H-Pyrano[3',7]indolizino[1,2-b]quinoline- 3,14(4H,12H)-dione, 10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-, (4S)-