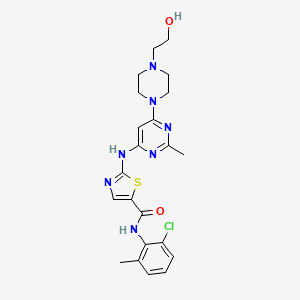
Dasatinib, 302962-49-8, Sprycel, BMS-354825, Dasatinib anhydrous, BMS 354825, Dasatinib (anhydrous), BMS354825, dasatinibum, N-(2-CHLORO-6-METHYLPHENYL)-2-({6-[4-(2-HYDROXYETHYL)PIPERAZIN-1-YL]-2-METHYLPYRIMIDIN-4-YL}AMINO)-1,3-THIAZOLE-5-CARBOXAMIDE, anh. dasatinib, N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)amino)thiazole-5-carboxamide, N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide, anhydrous dasatinib, Dasatinib (BMS-354825), dasatinib (anh.), N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide, BMS Dasatinib, UNII-X78UG0A0RN, X78UG0A0RN, DTXSID4040979, CHEBI:49375, NSC732517, NSC-759877, CHEMBL1421, DTXCID2020979, BMS-354825 HYDRATE, BMS 345825, NSC-732517, 5-Thiazolecarboxamide, N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)-1-piperazinyl)-2-methyl-4-pyrimidinyl)amino)-, 5-Thiazolecarboxamide, N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-, NCGC00181129-01, BMS 35482513, BMS-354825-03, UNII-RBZ1571X5H, CHEBI:70839, C22H26ClN7O2S, DTXSID50235486, n-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)amino)-1,3-thiazole-5-carboxamide, SMR002529551, CAS-302962-49-8, NSC 759877, 302962-49-8 pound not863127-77-9, Dasatinib [USAN:INN], 1N1, Kinome_3650, Dasatinib (JAN/INN), DASATINIB [INN], DASATINIB [MI], Sprycel (Bristol Meyers), DASATINIB [WHO-DD], SCHEMBL8226, Dasatinib,BMS-354825, MLS003915609, MLS004774145, MLS006010904, GTPL5678, BDBM13216, cid_3062316, Dasatinib (BMS-354825)?, DTXCID50157977, EX-A401, L01XE06, 5-Thiazolecarboxamide, monohydrate, BCPP000263, HMS2043N05, HMS3244A05, HMS3244A06, HMS3244B05, HMS3265C19, HMS3265C20, HMS3265D19, HMS3265D20, HMS3654K05, HMS3744C11, Pharmakon1600-01502275, BCP01797, Tox21_112736, MFCD11046566, NSC759877, NSC800087, s1021, AKOS015902363, Tox21_112736_1, BCP9000589, CCG-264779, CS-0100, DB01254, GS-6548, NSC-800087, SB17284, NCGC00181129-02, NCGC00181129-03, NCGC00181129-05, NCGC00181129-06, NCGC00181129-07, NCGC00181129-12, NCGC00181129-14, NCGC00181129-22, NCGC00481571-01, 2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide, AC-22749, BCB03_000715, HY-10181, N-(2-chloro-6-methyl-phenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methyl-pyrimidin-4-yl]amino]thiazole-5-carboxamide, AM20080877, D5949, FT-0650671, NS00001968, PA-3062316, SW208076-5, D-3307, D03658, EN300-123025, AB01273956-01, AB01273956-02, AB01273956_03, AR-270/43507994, Q419940, SR-00000000554, Q-101345, SR-00000000554-5, BRD-K49328571-001-05-1, BRD-K49328571-001-07-7, Z1546610486, N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl}amino)-1,3-thiazole-5-carboxamide, N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide