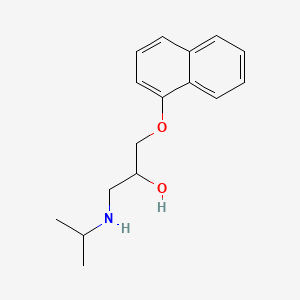
propranolol, Propanolol, 525-66-6, beta-Propranolol, Betalong, Euprovasin, Proprasylyt, Reducor, Propanalol, Anapriline, Propanix, propranololo, Corpendol, Sawatal, dl-propranolol, Sumial, Inderal, Racemic propranolol, Propranololum, D,L-Propranolol, (+-)-Propranolol, racemic-Propranolol, .beta.-Propranolol, 1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol, CCRIS 3082, 1-((1-Methylethyl)amino)-3-(1-naphthalenyloxy)-2-propanol, 1-(isopropylamino)-3-(1-naphthyloxy)propan-2-ol, Propanolol [INN-Spanish], UNII-9Y8NXQ24VQ, Propranololum [INN-Latin], EINECS 208-378-0, EINECS 235-867-6, 9Y8NXQ24VQ, hemangiol, 1-Isopropylamino-3-(1-naphthyloxy)-2-propanol, Bedranol, CHEBI:8499, 2-Propanol, 1-((1-methylethyl)amino)-3-(1-naphthalenyloxy)-, (+/-)-propranolol, 1-Isopropylamino-3-(naphthalen-1-yloxy)-propan-2-ol, 2-Propanol, 1-(isopropylamino)-3-(1-naphthyloxy)-, CHEMBL27, rac-Propanolol, AY-64043-, Betadren, DTXSID6023525, 2-Propanol, 1-(isopropylamino)-3-(1-naphthyloxy)-, (+-)-, 2-Propanol, 1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-, Inderex, Inderol, Pranolol, Propranolol (INN), (1)-1-(Isopropylamino)-3-(naphthyloxy)propan-2-ol, 13013-17-7, 1-(naphthalen-1-yloxy)-3-(propan-2-ylamino)propan-2-ol, 1-(naphthalen-1-yloxy)-3-[(propan-2-yl)amino]propan-2-ol, PROPRANOLOL [INN], 2-Propanol, 1-((1-methylethyl)amino)-3-(1-naphthalenyloxy)-, (+-)-, Inderal hydrochloride, Propanolol (INN-Spanish), Propranololum (INN-Latin), Propranololo [DCIT], 3-(naphthalen-1-yloxy)-1-(propan-2-ylamino)propan-2-ol, Propranalol, Propranolol [INN:BAN], AY 20694, b-Propranolol, Propranolol (TN), Dociton (Salt/Mix), Inderal (Salt/Mix), Obsidan (Salt/Mix), Propanolol,(+/-), PROPRANOLOL, d, Avlocardyl (Salt/Mix), beta-Propranolol;Dociton, PROPANOLOL(-), PROPRANOLOL [MI], Prestwick0_000952, Prestwick1_000952, Prestwick2_000952, Prestwick3_000952, PROPRANOLOL,(+), PROPRANOLOL,(-), Spectrum2_001301, Spectrum2_001699, Spectrum3_000883, Spectrum3_001071, Spectrum4_000974, Spectrum4_001222, Spectrum5_000751, (.+/-.)-Propranolol, PROPRANOLOL [VANDF], SCHEMBL3955, 3-[(methylethyl)amino]-1-naphthyloxypropan-2-ol, Lopac0_000896, Oprea1_304193, BSPBio_000944, BSPBio_002682, CBDivE_006180, GTPL564, KBioGR_001347, KBioGR_001684, KBioGR_002515, KBioSS_002523, PROPRANOLOL [WHO-DD], DivK1c_000023, SPBio_001361, SPBio_001658, SPBio_003093, (+)-Propranolol hydrochloride; (R)-(+)-Propranolol hydrochloride, 1-(Isopropylamino)-3-(naphthalen-1-yloxy)propan-2-ol, BPBio1_001040, DTXCID903525, ICI 45520 (Salt/Mix), NSC 91523 (Salt/Mix), SCHEMBL12264958, BDBM25761, HY-B0573B, KBio1_000023, KBio2_002515, KBio2_005083, KBio2_007651, KBio3_001766, KBio3_001902, KBio3_002993, C07AA05, cMAP_000071, NINDS_000023, (A+/-)-Propranolol hydrochloride, [2-hydroxy-3-(naphthalen-1-yloxy)propyl](propan-2-yl)amine, Bio1_000367, Bio1_000856, Bio1_001345, HMS2090L21, HMS3428G03, BCP26001, BCP31343, BBL023437, PDSP1_000767, PDSP1_001607, PDSP1_001608, PDSP2_000755, PDSP2_001591, PDSP2_001592, STK735510, AKOS000588816, AKOS016050338, CCG-103643, DB00571, FE-0204, SDCCGSBI-0050871.P004, IDI1_000023, NCGC00015798-04, NCGC00015798-05, NCGC00015798-06, NCGC00015798-07, NCGC00015798-08, NCGC00015798-09, NCGC00015798-15, NCGC00015798-19, NCGC00024690-02, NCGC00024690-03, SBI-0050871.P003, AB00053537, CS-0069968, NS00000196, EN300-40731, C07407, D08443, G60902, AB00053537-10, AB00053537_11, AB00053537_12, 1-(Isopropylamino)-3-(1-naphthoxy)-propan-2-ol, L000679, Q423364, 1(-alpha-naphthoxy)-3-(iso-propylamino)-2-propanol, 1-(alpha-naphthoxy)-3-(iso-propylamino)-2-propanol, 1-(alpha-naphthoxy)-3-(isopropylamino)-2-propanol, 1-isopropylamino-3-(naphthalen-1-yloxy)propan-2-ol, W-109550, 1-(isopropylamino)-3-naphthalen-1-yloxy-propan-2-ol, BRD-A10070317-003-06-9, BRD-A10070317-003-17-6, 1-((1-Methylethyl)amino)-3-(1-naphthyloxy)-2-propanol, 1-(1-Naphthyloxy)-2-hydroxy-3-(isopropylamino)propane, F0001-3681, 1-[(1-methylethyl)amino]-3-(naphthalen-1-yloxy)propan-2-ol