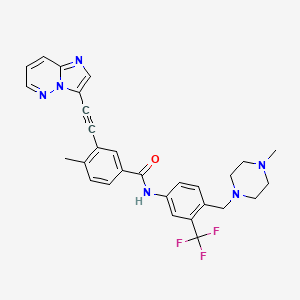
PONATINIB, 943319-70-8, AP24534, Ponatinib (AP24534), AP 24534, 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide, ponatinibum, AP-24534, UNII-4340891KFS, CHEBI:78543, HSDB 8184, 3-(2-Imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]benzamide, 4340891KFS, CHEMBL1171837, DTXSID50241426, 943319-70-8 (free base), 3-(2-imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]benzamide, 3-(Imidazo[1,2-B]pyridazin-3-Ylethynyl)-4-Methyl-N-{4-[(4-Methylpiperazin-1-Yl)methyl]-3-(Trifluoromethyl)phenyl}benzamide, Benzamide, 3-(2-imidazo(1,2-b)pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methyl-1- piperazinyl)methyl)-3-(trifluoromethyl)phenyl)-, AP24534(Ponatinib), 3-(2-(Imidazo(1,2-b)pyridazin-3-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1- yl)methyl)-3-(trifluoromethyl)phenyl)benzamide, 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide., Ponatinib [USAN], Ponatinib [USAN:INN], 0LI, 3-(2-(IMIDAZO(1,2-B)PYRIDAZIN-3-YL)ETHYNYL)-4-METHYL-N-(4-((4-METHYLPIPERAZIN-1-YL)METHYL)-3-(TRIFLUOROMETHYL)PHENYL)BENZAMIDE, 3-(2-(Imidazo[1,2-b]pyridazin-3-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide, 3-(2-{imidazo[1,2-b]pyridazin-3-yl}ethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide, 3-(imidazo(1,2-b)pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide, 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzam ide, 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-n-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide, BENZAMIDE, 3-(2-IMIDAZO(1,2-B)PYRIDAZIN-3-YLETHYNYL)-4-METHYL-N-(4-((4-METHYL-1-PIPERAZINYL)METHYL)-3-(TRIFLUOROMETHYL)PHENYL)-, BENZAMIDE, 3-(2-IMIDAZO[1,2-B]PYRIDAZIN-3-YLETHYNYL)-4-METHYL-N-[4-[(4-METHYL-1-PIPERAZINYL)METHYL]-3-(TRIFLUOROMETHYL)PHENYL]-, Benzamide, 3-(2-imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]-; 3-(2-Imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]benzamide; AP 24534; Iclusig; Ponatinib, Ponatinib, Free Base, AP24534,Ponatinib, PONATINIB [INN], PONATINIB [MI], Ponatinib (USAN/INN), PONATINIB [VANDF], AP24534 (Ponatinib), PONATINIB [WHO-DD], MLS006010166, SCHEMBL589260, GTPL5890, DTXCID50163917, EX-A067, L01XE24, BCPP000397, HMS3295I23, HMS3654H16, BCP02037, BDBM50322535, MFCD17215203, NSC758487, NSC800855, AKOS015995214, AM81261, BCP9000307, CCG-264900, CS-0204, DB08901, NSC-758487, NSC-800855, PB34916, NCGC00263152-01, NCGC00263152-02, NCGC00263152-12, NCGC00263152-14, 3-(2-(imidazo(1,2-b)pyridazin-3-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-y-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide, AC-26973, AS-19133, HY-12047, SMR004701274, FT-0660376, NS00072196, S1490, SW218091-2, A24930, D09950, AB01565847_03, EN300-6733072, Q198728, BRD-K44227013-001-02-3, Z1828098700, 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzam, Benzamide, 3-(2-Imidazo(1,2-B)Pyridazin-3-Ylethynyl)-4-Methyl-N-(4-((4-Methyl-1-Piperazinyl)Methyl)-3-(Trifluoromethyl)Phenyl), Ponatinib;3-(2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide