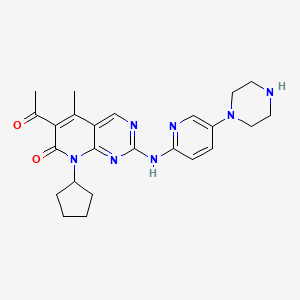
Palbociclib, 571190-30-2, PD-0332991, Ibrance, PD0332991, PD 0332991, UNII-G9ZF61LE7G, 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one, Palbociclib free base, G9ZF61LE7G, CHEBI:85993, PD 332991, 571190-30-2 (free base), MFCD11840850, 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-ylpyridin-2-ylamino)-8H-pyrido(2,3-d)pyrimidin-7-one, 6-ACETYL-8-CYCLOPENTYL-5-METHYL-2-[(5-PIPERAZIN-1-YLPYRIDIN-2-YL)AMINO]PYRIDO[2,3-D]PYRIMIDIN-7(8H)-ONE, 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)pyridin-2-yl]amino]-8H-pyrido[2,3-d]pyrimidin-7-one, Palbociclib(PD0332991), LQQ, Pyrido(2,3-d)pyrimidin-7(8H)-one, 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(1-piperazinyl)-2-pyridinyl)amino)-, 2euf, 6-ACETYL-8-CYCLOPENTYL-5-METHYL-2-((5-(PIPERAZIN-1-YL)PYRIDIN-2-YL)AMINO(PYRIDO(2,3-D)PYRIMIDIN-7(8H)-ONE, 6-ACETYL-8-CYCLOPENTYL-5-METHYL-2-((5-(PIPERAZIN-1-YL)PYRIDIN-2-YL)AMINO)-8H-PYRIDO(2,3-D)PYRIMIDIN-7-ONE, 6-acetyl-8-cyclopentyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}pyrido[2,3-d]pyrimidin-7(8H)-one, Palbociclib [USAN], PD-332991, 571190-30-2 pound not827022-32-2, Palbociclib [USAN:INN], palbociclibum, [d8]-Palbociclib, Ibrance (TN), Palbociclib- Bio-X, Kinome_3823, Kinome_3824, PALBOCICLIB [MI], 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8h-pyrido[2,3-d]pyrimidin-7-one hydrochloride, PALBOCICLIB [INN], PALBOCICLIB [JAN], Palbociclib (JAN/USAN), PALBOCICLIB [WHO-DD], 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one, SCHEMBL462630, BDBM6309, CHEMBL189963, GTPL7380, PD 0332991 (Palbociclib), DTXSID40972590, EX-A408, L01XE33, PALBOCICLIB [ORANGE BOOK], 2euf; PD 0332991, AHJRHEGDXFFMBM-UHFFFAOYSA-N, OTAVA-BB 1115529, BCPP000125, HMS3265M09, HMS3265M10, HMS3265N09, HMS3265N10, HMS3744G13, AMY14886, BCP09274, BCP18381, NSC758247, NSC772256, NSC800815, PD 991, s4482, AKOS022205241, BCP9001058, DB09073, NSC-758247, NSC-772256, NSC-800815, SB40426, Pyrido-[2,3-d]-pyrimidin-7-one 43, NCGC00263129-01, NCGC00263129-08, NCGC00263129-21, NCGC00263129-22, 6-acetyl-8-cyclopentyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H,8H-pyrido[2,3-d]pyrimidin-7-one, AC-25485, AS-17016, BP166224, HY-50767, SY026143, A8153, NS00069617, D10372, EN300-18531248, PD 0332991,PD0332991, BRD-K51313569-001-01-1, Q15269707, Z2216894329, 6-acetyl-8-cyclopentyl-5-methyl-2-(5-(piperazin-1-yl)pyridin-2-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one, 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8H-pyrido[2,3-d]pyrimidin-7-one, 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[6,5-d]pyrimidin-7-one, 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]pyrido[2,3-d]pyrimidin-7(8H)-one, 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)-pyridin-2-yl]amino]-8H-pyrido[2,3-d]pyrimidin-7-one, 8-cyclopentyl-6-acetyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H,8H-pyrido[2,3-d]pyrimidin-7-one