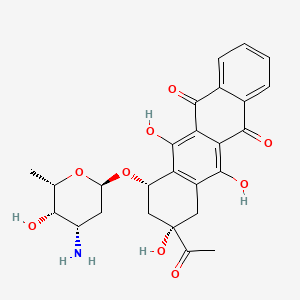
IDARUBICIN, 58957-92-9, 4-Demethoxydaunorubicin, 4-Demethoxydaunomycin, Idarubicina, Idarubicine, Idarubicinum, Idarubicine [INN-French], Idarubicinum [INN-Latin], Idarubicina [INN-Spanish], Daunomycin, 4-demethoxy-, CCRIS 5083, UNII-ZRP63D75JW, ZRP63D75JW, Idarubicin (INN), CHEBI:42068, NSC 256439, (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione, 5,12-Naphthacenedione, 9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S-cis)-, DTXSID7023142, IDARUBICIN [INN], 4-Desmethoxydaunorubicin, Idarubicine (INN-French), Idarubicinum (INN-Latin), Idarubicin [INN:BAN], Idarubicina (INN-Spanish), (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside, (7S,9S)-9-acetyl-7-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,9,11-trihydroxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione, 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)-, DM5, 4 Demethoxydaunorubicin, MLS001401448, NSC256439, Zavedos (TN), 4 Desmethoxydaunorubicin, NCGC00093976-03, SMR000466355, 4-DMD, Idarubicin?, SR-01000075934, IDARUBICIN [MI], (7S-cis)-9-Acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-5,12-naphthacenedione, IDARUBICIN [VANDF], 4-DMDR, I 1656, SCHEMBL3750, CHEMBL1117, IDARUBICIN [WHO-DD], Lopac0_000600, KBioSS_002388, Idarubicin hydrochloride, solid, cid_636362, DTXCID503142, GTPL7083, 4-DEMETHOXY-DAUNORUBICIN, BDBM58490, L01DB06, BCPP000207, HMS2089D05, HMS3261H22, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride, Tox21_500600, HY-17381A, AKOS015895563, AC-9384, BCP9000773, CCG-204689, DB01177, LP00600, SDCCGSBI-0050582.P002, NCGC00093976-01, NCGC00093976-02, NCGC00093976-04, NCGC00093976-05, NCGC00093976-18, NCGC00261285-01, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione, CS-0007534, EU-0100600, D08062, AB00698511-06, AB00698511-08, AB00698511-09, AB00698511-10, AB00698511_11, EN300-7479233, A832088, A935911, Q1063862, SR-01000075934-1, BRD-K69650333-001-01-1, BRD-K69650333-001-02-9, BRD-K69650333-003-14-0, Idarubicin, United States Pharmacopeia (USP) Reference Standard, (1S,3S)-3-ACETYL-1,2,3,4,6,11-HEXAHYDRO-3,5,12-TRIHYDROXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSIDE, (1S,3S)-3-ACETYL-1,2,3,4,6,11-HEXAHYDRO-3,5,12-TRIHYDROXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-alpha-L-LYXO-HEXOPYRANOSIDE, (1S,3S)-3-Acetyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-6,11-dioxo-1-naphthacenyl-3-amino-2,3,6-trideoxy-alpha-L-hexopyranoside, (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione, (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride, (7S,9S)-9-Acetyl-7-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione, (7S,9S)-9-acetyl-7-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione, (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride, (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione, (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride, 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)-, 5,12-NAPHTHACENEDIONE, 9-ACETYL-7-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSYL)OXY)-7,8,9,10-TETRAHYDRO-6,9,11-TRIHYDROXY-, (7S,9S)-, 5,12-NAPHTHACENEDIONE, 9-ACETYL-7-((3-AMINO-2,3,6-TRIDEOXY-alpha-L-LYXO-HEXOPYRANOSYL)OXY)-7,8,9,10-TETRAHYDRO-6,9,11-TRIHYDROXY-, (7S,9S)-