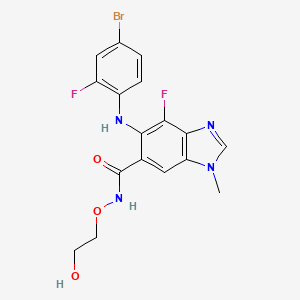
Binimetinib, 606143-89-9, MEK162, Mektovi, ARRY-162, ARRY-438162, MEK-162, 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide, NVP-MEK162, binimetinibum, ARRY 162, Binimetinib (MEK-162), ARRY 438162, MEK162 (ARRY-162, ARRY-438162), UNII-181R97MR71, MEK162(Binimetinib), 181R97MR71, CHEBI:145371, MFCD22124525, Binimetinib (MEK162, ARRY-162, ARRY-438162), 5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-6-carboxamide, 6-(4-bromo-2-fluoroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide, 4-Quinazolinamine, N-(3,4-dichloro-2-fluorophenyl)-6-methoxy-7-[[(3aa,5a,6aa)-octahydro- 2-methylcyclopenta[c]pyrrol-5-yl]methoxy]-, 5-(4-Bromo-2-fluoroanilino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide, MEK 162, 5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-6-carboxamide., 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-1,3-benzodiazole-6-carboxamide, 6-[(4-bromo-2-fluorophenyl)amino]-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide, N-(2-hydroxyethoxy)-4-fluoro-5-(2-fluoro-4-bromophenylamino)-1-methyl-1H-benzoimidazole-6-carboxamide, Binimetinib [USAN:INN], C17H15BrF2N4O3, 1H-BENZIMIDAZOLE-6-CARBOXAMIDE, 5-((4-BROMO-2-FLUOROPHENYL)AMINO)-4-FLUORO-N-(2-HYDROXYETHOXY)-1-METHYL-, 1H-Benzimidazole-6-carboxamide, 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-, 1H-Benzimidazole-6-carboxamide, 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-; 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide; ARRY 162; ARRY 438162; MEK 162, 5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-1,3-benzodiazole-6-carboxamide, 5-((4-BROMO-2-FLUOROPHENYL)AMINO)-4-FLUORO-N-(2-HYDROXYETHOXY)-1-METHYL-1H-BENZIMIDAZOLE-6-CARBOXAMIDE, 5-(4-BROMO-2-FLUOROPHENYLAMINO)-4-FLUORO-N-(2-HYDROXYETHOXY)-1-METHYL-1H-BENZO(D)IMIDAZOLE-6-CARBOXAMIDE, 5-(4-Bromo-2-fluorophenylamino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-6-carboxamide, Mektovi (TN), ARRY-162; ARRY-438162; MEK 162; ARRY 162; ARRY 438162, Binimetinib; Mek162, BINIMETINIB [MI], Binimetinib (MEK162), BINIMETINIB [INN], BINIMETINIB [JAN], Binimetinib (JAN/USAN), BINIMETINIB [USAN], BINIMETINIB [WHO-DD], MLS006011180, SCHEMBL570088, GTPL7921, CHEMBL3187723, AMY9056, BINIMETINIB [ORANGE BOOK], DTXSID70209422, L01XE41, ACWZRVQXLIRSDF-UHFFFAOYSA-N, ARRY-162,MEK-162, BDBM520649, GLXC-04704, HMS3652J14, HMS3747G09, BCP06780, EX-A1024, NSC764042, NSC788187, NSC799361, s7007, AKOS026750517, CCG-269133, CS-0627, DB11967, NSC-764042, NSC-788187, NSC-799361, SB16501, NCGC00345804-01, NCGC00345804-10, 1073666-70-2, AC-29023, AS-16706, DA-35030, HY-15202, SMR004702949, SY284756, cas:606143-89-9;MEK162, FT-0697088, NS00072273, SW219910-1, D10604, EN300-7411873, Binimetinib;MEK-162; ARRY-162;ARRY-438162, J-516581, Q19903515, US11147816, Binimetinib (ARRY-162, ARRY-438162), 5-((4-BROMO-2-FLUOROPHENYLAMINO)-4-FLUORO-N-(2-HYDROXYETHOXY)-1-METHYL-1H-BENZO (D) IMIDAZOLE-6-CARBOXAMIDE, 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methylbenzimidazole-6-carboxamide, 6-(4-bromo-2-fluorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxyethyoxy)-amide, QO7