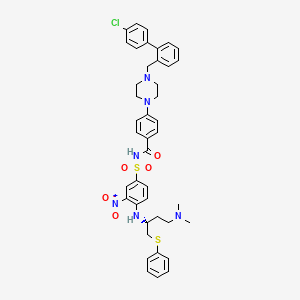
ABT-737, 852808-04-9, ABT 737, ABT737, UNII-Z5NFR173NV, 4-[4-[(4'-Chloro[1,1'-biphenyl]-2-yl)methyl]-1-piperazinyl]-N-[[4-[[(1R)-3-(dimethylamino)-1-[(phenylthio)methyl]propyl]amino]-3-nitrophenyl]sulfonyl]benzamide, Z5NFR173NV, (R)-4-(4-((4'-chloro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((4-((4-(dimethylamino)-1-(phenylthio)butan-2-yl)amino)-3-nitrophenyl)sulfonyl)benzamide, CHEMBL376408, 4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-(dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl]sulfonylbenzamide, 4-{4-[(4'-chlorobiphenyl-2-yl)methyl]piperazin-1-yl}-N-{[4-({(1R)-3-(dimethylamino)-1-[(phenylsulfanyl)methyl]propyl}amino)-3-nitrophenyl]sulfonyl}benzamide, 4-{4-[(4'-Chlorobiphenyl-2-Yl)methyl]piperazin-1-Yl}-N-{[4-({(1r)-3-(Dimethylamino)-1-[(Phenylthio)methyl]propyl}amino)-3-Nitrophenyl]sulfonyl}benzamide, C42H45ClN6O5S2, 2yxj, 4-[4-({4'-CHLORO-[1,1'-BIPHENYL]-2-YL}METHYL)PIPERAZIN-1-YL]-N-(4-{[(2R)-4-(DIMETHYLAMINO)-1-(PHENYLSULFANYL)BUTAN-2-YL]AMINO}-3-NITROBENZENESULFONYL)BENZAMIDE, BENZAMIDE, 4-(4-((4'-CHLORO(1,1'-BIPHENYL)-2-YL)METHYL)-1-PIPERAZINYL)-N-((4-(((1R)-3-(DIMETHYLAMINO)-1-((PHENYLTHIO)METHYL)PROPYL)AMINO)-3-NITROPHENYL)SULFONYL)-, Benzamide, 4-[4-[(4'-chloro[1,1'-biphenyl]-2-yl)methyl]-1-piperazinyl]-N-[[4-[[(1R)-3-(dimethylamino)-1-[(phenylthio)methyl]propyl]amino]-3-nitrophenyl]sulfonyl]-, N3C, SCHEMBL158942, GTPL8320, ABT 737 [WHO-DD], DTXSID7042641, N-Benylpiperazine derivative, 2, BDBM21447, CHEBI:47575, EX-A056, HPLNQCPCUACXLM-PGUFJCEWSA-N, GLXC-04997, MFCD12756212, NSC758873, s1002, compound 2 [PMID 17256834], AKOS016003299, AM81254, CCG-264769, CS-0014, NSC-758873, NCGC00253562-01, NCGC00253562-02, NCGC00253562-12, BP-25381, HY-50907, SW218108-2, BRD-K56301217-001-01-7, Q25105040, 4-(4-((4'-chloro-1,1'-biphenyl-2-yl)methyl)-1-piperazinyl)-N-((4-(((1R)-3-(dimethylamino)-1-((phenylsulfanyl)methyl)propyl)amino)-3-nitrophenyl)sulfony)benzamide, 4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1-yl)-N-[(4-{[(2R)-4-(dimethylamino)-1-(phenylsulfanyl)butan-2-yl]amino}-3-nitrobenzene)sulfonyl]benzamide, 4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4- (dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl] sulfonylbenzamide, 4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-dimethylamino-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl]sulfonylbenzamide, 4-{4-[(4'-chloro[1,1'-biphenyl]-2-yl)methyl]piperazin-1-yl}-N-(4-{[(2R)-4-(dimethylamino)-1-(phenylsulfanyl)butan-2-yl]amino}-3-nitrobenzene-1-sulfonyl)benzamide, 4-{4-[(4'-chloro[biphenyl]-2-yl)methyl]piperazin-1-yl}-N-[(4-{[(2R)-4-(dimethylamino)-1-(phenylsulfanyl)butan-2-yl]amino}-3-nitrophenyl)sulfonyl]benzamide