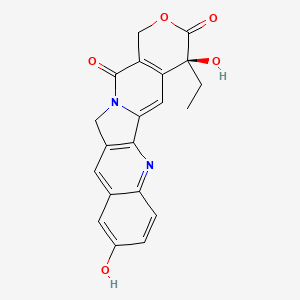
10-Hydroxycamptothecin, 19685-09-7, (S)-10-Hydroxycamptothecin, Hydroxycamptothecin, 10-hydroxycamptothecine, 10-Hydroxy camptothecin, (S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, Hydroxycamptothecine, (4S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, Camptothecin, hydroxy-, Camptothecine, 10-hydroxy-, 10-HCPT, 10-Hydroxy-Camptothecin, NSC107124, NSC-107124, CHEMBL273862, CHEBI:81395, 9Z01632KRV, Hydroxy camptothecine, MFCD02093100, (S)-4-ethyl-4,9-dihydroxy-1,12-dihydro-14H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H)-dione, Camptothecin, 10-hydroxy-, (20S)-4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione, (S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione ((S)-10-Hydroxycamptothecin), NSC 107124, (S)-10-Hydroxycamptothecin hydrate, UNII-9Z01632KRV, CAMPTOTHECIN, 10-HYDROXY, 10-Hydroxy-CPT, Spectrum_001639, (+)-(S)-10-HYDROXYCAMPTOTHECIN, SpecPlus_000763, Spectrum2_001660, Spectrum3_001621, Spectrum4_001815, Spectrum5_000549, ethyl(dihydroxy)[?]dione, SCHEMBL25875, BSPBio_003281, KBioGR_002454, KBioSS_002119, 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione, 4-ethyl-4,9-dihydroxy-, hydrate, (S)-, DivK1c_006859, SPECTRUM1504123, SPBio_001819, KBio1_001803, KBio2_002119, KBio2_004687, KBio2_007255, KBio3_002501, DTXSID00941444, EX-A988, BCP01385, HY-N0095, (+)-10-HYDROXYCAMPTOTHECIN, BDBM50008922, CCG-38770, s2423, s3898, AKOS015919293, AC-5502, BCP9000058, CS-5193, DB12385, 10-HYDROXYCAMPTOTHECIN [WHO-DD], NCGC00095986-01, NCGC00095986-02, NCGC00095986-03, NCGC00095986-04, NCGC00178165-01, 1H-Pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione-,4-ethyl-4,9-dihydroxy-, (S)-, AC-13221, AS-13196, NCI60_000173, SY010687, FT-0607192, FT-0689364, H1463, NS00018317, A25382, C17939, EN300-19810684, SR-05000002620, Q-100241, SR-05000002620-1, BRD-K63784565-001-02-1, BRD-K63784565-001-03-9, Q27155328, Z3093896188, 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione, (19S)-19-ethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaene-14,18-dione, (19S)-19-ethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaene-14,18-dione, (4S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, (S)-10-Hydroxycamptothecin;-;(+/-)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione, (S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3 inverted exclamation mark ,4 inverted exclamation mark :6,7]indolizino[1,2-b]quinoline-3,14-(4H,12H)-dione, (S)-4-Ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-(4H,12H)-dione, 1H-PYRANO(3',4':6,7)INDOLIZINO(1,2-B)QUINOLINE-3,14(4H,12H)-DIONE, 4-ETHYL-4,9-DIHYDROXY-, (4S)-, 1H-Pyrano[3',7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione-,4-ethyl-4,9-dihydroxy-, (S)-, 4-Ethyl-4,10-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione, 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (10-hydroxycamptothecin)