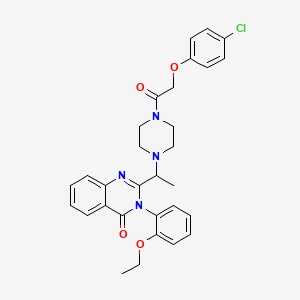
ERASTIN, 571203-78-6, 2-[1-[4-[2-(4-chlorophenoxy)acetyl]-1-piperazinyl]ethyl]-3-(2-ethoxyphenyl)-4(3H)-Quinazolinone, 2-(1-(4-(2-(4-chlorophenoxy)acetyl)piperazin-1-yl)ethyl)-3-(2-ethoxyphenyl)quinazolin-4(3H)-one, MFCD09837984, 2-[1-[4-[2-(4-chlorophenoxy)acetyl]piperazin-1-yl]ethyl]-3-(2-ethoxyphenyl)quinazolin-4-one, 2-(1-(4-(2-(4-Chlorophenoxy)acetyl)-1-piperazinyl)ethyl)-3-(2-ethoxyphenyl)-4(3H)-quinazolinone, 2-(1-(4-(2-(4-Chlorophenoxy)acetyl)piperazin-1-yl)ethyl)-3-(2-ethoxyphenyl)quinazolin-4-one, 2-[1-[4-[2-(4-Chlorophenoxy)acetyl]-1-piperazinyl]ethyl]-3-(2-ethoxyphenyl)-4(3H)-quinazolinone;, ZJA3NS42T9, CHEMBL401989, SCHEMBL4457820, Erastin, >=98% (HPLC), CHEBI:94287, DTXSID80458949, EX-A295, HMS3653K21, HMS3868M03, BCP27907, WXA20378, BDBM50376126, s7242, AKOS025147365, CCG-269987, CS-1675, Erastin - CAS 571203-78-6, SB19588, NCGC00351608-10, NCGC00351608-14, AC-35446, AS-55898, DA-42059, HY-15763, SY345515, E7781, FT-0700333, SW208651-2, C21478, E-7781, A869751, BRD-A25004090-001-01-9, BRD-A25004090-001-02-7, BRD-A25004090-001-06-8, Q27166099, 2-[1-[4-[2-(4-chlorophenoxy)-1-oxoethyl]-1-piperazinyl]ethyl]-3-(2-ethoxyphenyl)-4-quinazolinone, 2-[1-[4-[2-(4-Chlorophenoxy)acetyl]-1-piperazinyl]ethyl]-3-(2-ethoxyphenyl)quinazolin-4(3H)-one, 4(3H)-Quinazolinone, 2-[1-[4-[2-(4-chlorophenoxy)acetyl]-1-piperazinyl]ethyl]-3-(2-ethoxyphenyl)-, Piperazine, 1-((4-chlorophenoxy)acetyl)-4-(1-(3-(2-ethoxyphenyl)-3,4-dihydro-4-oxo-2-quinazolinyl)ethyl)-