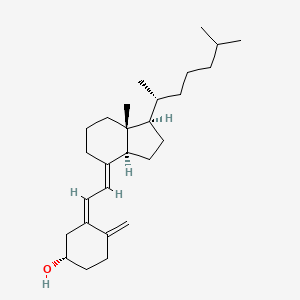
Vitamin D3, cholecalciferol, 67-97-0, Colecalciferol, Calciol, Ricketon, Oleovitamin D3, Deparal, Arachitol, Delsterol, Trivitan, Ebivit, Vigantol, Vigorsan, vitamin d-3, Colecalcipherol, Colecalciferolum, Cholecalciferolum, D3-Vicotrat, D3-Vigantol, Vi-de-3-hydrosol, NEO Dohyfral D3, Provitina, Quintox, Rampage, 1406-16-2, Vitinc Dan-Dee-3, (+)-Vitamin D3, Cholecalciferol, D3, Colecalciferolo, Vi-De3, Duphafral D3 1000, Delta-D, Colecalciferol D3, Irradiated 7-dehydrocholesterol, CCRIS 6286, HSDB 820, Videkhol, 7-Dehydrocholesterol, irradiated, FeraCol, CHEBI:28940, Granuvit D3, CC, EINECS 200-673-2, Colecalciferolum [INN-Latin], EPA Pesticide Chemical Code 202901, NSC 375571, NSC-375571, UNII-1C6V77QF41, VIGANTOLETTEN, Colecalciferol [INN], 1C6V77QF41, (3beta,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-3-ol, MFCD00078131, (1S,3Z)-3-[(2E)-2-[(1R,3aS,7aR)-7a-methyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol, Vitamin d (cholecalciferol), 9,10-Secocholesta-5,7,10(19)-trien-3-beta-ol, AK R215 COMPONENT COLECALCIFEROL, AK-R215 COMPONENT COLECALCIFEROL, 9,10-Seco(5Z,7E)-5,7,10(19)-cholestatrien-3-ol, Micro-dee, DTXSID6026294, Vitamin d3 (as cholecalciferol), (5Z,7E)-(3S)-9,10-secocholesta-5,7,10(19)-trien-3-ol, 9,10-Secocholesta-5,7,10(19)-trien-3-ol, (3beta,5Z,7E)-, Vitamin d assay system suitability, VidDe-3-hydrosol, Cholecalciferol [USP:BAN:JAN:ISO], NSC375571, Colecalciferol (INN), (3S,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-3-ol, Vitamin D3 10 microg/mL in Acetonitrile, (5Z,7E)-(3S)-9,10-seco-5,7,10(19)-cholestatrien-3-ol, 9,10-Secocholestra-5,7,10(19)-trien-3-ol, (3beta,5Z,7E)-, 9,10-Secocholesta-5,7,10(19)-trien-3-ol, Colecalciferolum (INN-Latin), COLECALCIFEROL (MART.), COLECALCIFEROL [MART.], CHOLECALCIFEROL (USP-RS), CHOLECALCIFEROL [USP-RS], 9,10-Seco(5Z,7E)-5,7,10(19)-cholestatrien-3beta-ol, Colecalciferolo [DCIT], 9,10-Secocholesta-5(Z),7(E),10(19)-trien-3(.beta.)-ol, 9,10-Secocholesta-5,7,10(19)-trien-3-ol, (3b,5Z,7E)-, 9,10-Secocholesta-5,7,10(19)-trien-3-ol, (3.beta.,5Z,7E)-, CHOLECALCIFEROL (EP MONOGRAPH), Cholecalciferol (USP:BAN:JAN:ISO), CHOLECALCIFEROL [EP MONOGRAPH], CHOLECALCIFEROL (USP MONOGRAPH), CHOLECALCIFEROL [USP MONOGRAPH], (1S,3Z)-3-[(2E)-2-[(1R,3aS,7aR)-1-[(1R)-1,5-dimethylhexyl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylene-cyclohexanol, (S,Z)-3-(2-((1R,3aS,7aR,E)-7a-methyl-1-((R)-6-methylheptan-2-yl)octahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexan-1-ol, Cyclohexanol, 3-[(2E)-2-[(1R,3aS,7aR)-1-[(1R)-1,5-dimethylhexyl]octahydro-7a-methyl-4H-inden-4-ylidene]ethylidene]-4-methylene-, (1S,3Z)-, Vitamin D 3, CCRIS 5813, Vitamin D3 emulsifiable, EINECS 215-797-2, DTXCID306294, UNII-9VU1KI44GP, Vitamin D3; Cholecalciferol, Devaron, Vitamin D3 (Cholecalciferol), (3.beta.,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-3-ol, vitaminum d3, DP-R206, Osteo-Vit3, Prestwick_63, Cholecalciferol D3, NCGC00159331-02, Cyclohexanol, 3-((2E)-2-((1R,3aS,7aR)-1-((1R)-1,5-dimethylhexyl)octahydro-7a-methyl-4H-inden-4-ylidene)ethylidene)-4-methylene-, (1S,3Z)-, 9,10-secocholesta-5,7,10-trien-3-ol, Novel-D3, DECALCITROL, ()-Vitamin D3, Cholecalciferol (D3), Delta-D (TN), VITA-DESIC, CHOLECALCIFEROL IMPURITY A (EP IMPURITY), Prestwick3_000429, bmse000507, UPCMLD-DP152, VITAMIN D3 [MI], VITAMIN D3 [FCC], SCHEMBL3126, 9VU1KI44GP, CHEMBL1042, BSPBio_000418, CHOLECALCIFEROL [ISO], CHOLECALCIFEROL [JAN], 9,10-Secocholesta-5,7,10(19)-trien-3beta-ol, Cholecalciferol; 67-97-0, Cholecalciferol (JP17/USP), CHOLECALCIFEROL [HSDB], CHOLECALCIFEROL [VANDF], BPBio1_000460, MEGxm0_000458, COLECALCIFEROL [WHO-DD], COLECALCIFEROL [WHO-IP], UPCMLD-DP152:001, ACon1_001997, A11CC05, COLECALCIFEROL [EMA EPAR], HMS2096E20, Cholecalciferol, >=98% (HPLC), (1S,3Z)-3-((2E)-2-((1R,3AR,7AS)-7A-METHYL-1-((2R)-6-METHYLHEPTAN-2-YL)-2,3,3A,5,6,7-HEXAHYDRO-1H-INDEN-4-YLIDENE)ETHYLIDENE)-4-METHYLIDENE-CYCLOHEXAN-1-OL, (1S,3Z)-3-[(2E)-2-[(1R,3AR,7AS)-7A-METHYL-1-[(2R)-6-METHYLHEPTAN-2-YL]-2,3,3A,5,6,7-HEXAHYDRO-1H-INDEN-4-YLIDENE]ETHYLIDENE]-4-METHYLIDENE-CYCLOHEXAN-1-OL, Cholecalciferol, analytical standard, GROWTH SUPPORTPATCH, HAUTUKI, BDBM50030475, CHOLECALCIFEROL [ORANGE BOOK], LMST03020001, s4063, Cholecalciferol for system suitability, 5,7-CHOLESTADIEN-3-BETAL-OL, AKOS015950641, AC-8884, CCG-268466, COLECALCIFEROLUM [WHO-IP LATIN], CS-1179, DB00169, DP-R206 COMPONENT VITAMIN D3, SMP1_000068, USEPA/OPP Pesticide Code: 202901, NCGC00091072-01, NCGC00159331-04, BS-42465, HY-15398, Cholecalciferol (D3), analytical standard, FT-0623718, NS00008889, C05443, D00188, FOSAMAX PLUS D COMPONENT CHOLECALCIFEROL, Cholecalciferol, meets USP testing specifications, Q139347, (5E,7E)-9,10-Secocholesta-5,7,10-trien-3-ol, Q-201931, VITAMIN D ASSAY SYSTEM SUITABILITY [USP-RS], 3-beta,Z,7E-9,10-Secocholestr-5,7,10(19)-trien-3-ol, (3beta,Z,7E)-9,10-Secocholesta-5,7,10(19)-trien-3-ol, Cholecalciferol, European Pharmacopoeia (EP) Reference Standard, Colecalciferol, British Pharmacopoeia (BP) Reference Standard, (5E,7E)-9,10-SECOCHOLESTA-5,7,10(19)-TRIEN-3beta-OL, Cholecalciferol, United States Pharmacopeia (USP) Reference Standard, Cholecalciferol for system suitability, European Pharmacopoeia (EP) Reference Standard, (1S,3Z)-3-[(2E)-2-[7a-Methyl-1-(6-methylheptan-2-yl)-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexan-1-ol, Cholecalciferol (Vitamin D3), Pharmaceutical Secondary Standard; Certified Reference Material