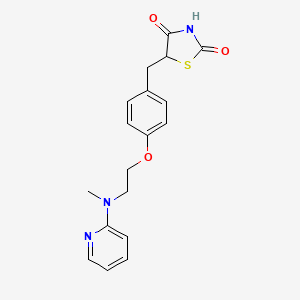
rosiglitazone, 122320-73-4, Avandia, Rosiglizole, 5-(4-(2-(Methyl(pyridin-2-yl)amino)ethoxy)benzyl)thiazolidine-2,4-dione, Brl-49653, Brl 49653, Rezult, Gaudil, Rosvel, BRL49653, TDZ 01, rosiglitazona, rosiglitazonum, Avandamet, Avandaryl, rosiglitazone (Avandia), DTXSID7037131, C18H19N3O3S, CHEBI:50122, UNII-05V02F2KDG, NSC-758698, 05V02F2KDG, TDZ-01, HSDB 7555, 2,4-Thiazolidinedione, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-, 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione, MFCD00871760, Rosigilitazone, 2,4-thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-, 5-((4-(2-Methyl-2-(pyridinylamino)ethoxy)phenyl)methyl)-2,4-thiazolidinedione-2-butenedioate, DTXCID5017131, 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione, Rosiglitazone (INN), NSC 758698, NCGC00095124-01, BRL 49653C, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-2,4-thiazolidinedione, 5-(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}benzyl)-1,3-thiazolidine-2,4-dione, ROSIGLITAZONE [INN], 5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione, ROSIGLITAZONE (IARC), ROSIGLITAZONE [IARC], ROSIGLITAZONE (MART.), ROSIGLITAZONE [MART.], 5-[4-[2-[Methyl(2-pyridyl)amino]ethoxy]benzyl]thiazolidine-2,4-dione, Rosiglitazone [INN:BAN], 5-[4-[2-(N-Methyl-N-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione, 5-(4-(2-(N-METHYL-N-(2-PYRIDINYL)AMINO)ETHOXY)BENZYL)-2,4-THIAZOLIDINEDIONE, CAS-122320-73-4, SR-01000763023, Rosiglitazon, Rosigltazone, Rosi, Rosiglitazone base, 5-(4-(2-(methyl(pyridin-2-yl)amino)ethoxy)benzyl)-1,3-thiazolidine-2,4-dione, 5-[4-[2-[N-methyl-N-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione, RGZ, Gaudil (TN), Rosiglitazone- Bio-X, Spectrum_001703, 1217260-35-9, Spectrum2_001241, Spectrum3_000997, Spectrum4_001125, Spectrum5_001464, ROSIGLITAZONE [MI], SCHEMBL5169, ROSIGLITAZONE [HSDB], BSPBio_002693, KBioGR_001609, KBioSS_002183, ROSIGLITAZONE [VANDF], SPECTRUM1504263, SPBio_001142, ROSIGLITAZONE [WHO-DD], GTPL1056, ROSIGLITAZONE [EMA EPAR], SCHEMBL14383595, KBio2_002183, KBio2_004751, KBio2_007319, KBio3_001913, A10BG02, Rosiglitazone, >=98% (HPLC), HMS1922J11, HMS2094O13, HMS3649G08, HMS3656K16, HMS3744M11, HMS3871L03, HMS3884N08, Pharmakon1600-01504263, BCP03047, Tox21_111434, BDBM50030474, CCG-39102, HB2556, NSC758698, STL350047, AKOS015894872, O-De-Me, 2-O-B-D-galactopyranoside, Tox21_111434_1, AC-3459, BCP9000017, CS-1088, DB00412, SB17326, NCGC00095124-02, NCGC00095124-03, NCGC00095124-04, NCGC00095124-05, NCGC00095124-06, NCGC00095124-08, BR164372, HY-17386, SY031184, BCP0726000232, NS00008920, R0106, S2556, SW197573-6, 6P-065, D08491, S00306, AB00698473-15, AB00698473-17, AB00698473-18, AB00698473-19, AB00698473_20, AB00698473_21, AB00698473_22, AB00698473_23, Q424771, Q-201681, SR-01000763023-5, SR-01000763023-6, BRD-A97437073-001-02-3, BRD-A97437073-001-03-1, BRD-A97437073-001-04-9, SR-01000763023-12, (+-)-5-(p-(2-(methyl-2-pyridylamino)ethoxy)benzyl)-2,4-thiazolidinedione, (RS)-5-{4-[2-(Methyl-2-pyridylamino)ethoxy]benzyl}-2,4-thiazolidinedion, 5-(4-(2-(Methyl(pyridin-2-yl)amino)ethoxy)-benzyl)thiazolidine-2,4-dione, 5-[4-[2-(N-Methyl-N-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4dione, IDMB (1uM BRL49653, 1uM Dexamethasone, 0.5uM IBMX, 10ug/mL Insulin), (+/-)-5-[p-[2-(methyl-2-pyridylamino)ethoxy]benzyl]-2,4-thiazolidinedione, 2,4-Thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]- (9CI), 5-[[4-[2-(methyl-(2-pyridyl)amino)ethoxy]phenyl]methyl] thiazolidine-2,4-dione, 5-[[4-[2-(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-2,4-thiazolidinedione, 5-[[4-[2-(Methyl-2-pyridinylamino)e thoxy]phenyl]methyl]-2,4-thiazolidinedione, 5-[[4-[2-(methyl-pyridin-2-ylamino)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione, 5-[4-[2-(N-methyl-N-(2-pyridyl)amino)ethoxy]benzyl] thiazolidine-2,4-dione, 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]phenyl methyl]thiazolidine-2,4-dione