


| Pair Name | Epigallocatechin gallate, Irinotecan | ||
| Phytochemical Name | Epigallocatechin gallate (PubChem CID: 65064 ) | ||
| Anticancer drug Name | Irinotecan (PubChem CID: 60838 ) | ||
| Structure of Phytochemical |
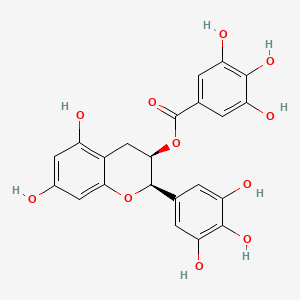
|
Download
2D
MOL
3D
MOL
|
|
| Structure of Anticancer Drug |
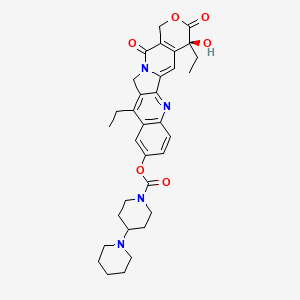
|
Download
2D
MOL
3D
MOL
|
|
| Pair Name | Epigallocatechin gallate, Irinotecan | |||
| Disease Info | [ICD-11: 2B91.Z] | Colorectal cancer | Investigative | |
| Biological Phenomena | Induction-->DNA damage | |||
| Gene Regulation | Down-regulation | Expression | TOP1 | hsa7150 |
| Down-regulation | Expression | CDK4 | hsa1019 | |
| Down-regulation | Expression | CCND1 | hsa595 | |
| Down-regulation | Expression | CCNB1 | hsa891 | |
| Up-regulation | Phosphorylation | RB1 | hsa5925 | |
| Up-regulation | Expression | MAP1LC3A | hsa84557 | |
| In Vitro Model | RKO | Colon carcinoma | Homo sapiens (Human) | CVCL_0504 |
| HCT 116 | Colon carcinoma | Homo sapiens (Human) | CVCL_0291 | |
| Result | EGCG synergizes the therapeutic effect of irinotecan through enhanced DNA damage in human colorectal cancer cells | |||
| Pair Name | Epigallocatechin gallate, Irinotecan | |||
| Disease Info | [ICD-11: 2B91.Z] | Colorectal cancer | Investigative | |
| Biological Phenomena | Induced-->GRP78-mediated endoplasmic reticulum stress | |||
| Gene Regulation | Down-regulation | Expression | BCL2 | hsa596 |
| Up-regulation | Expression | GRP78 | KEGG ID N.A. | |
| Down-regulation | Expression | ROS1 | hsa6098 | |
| In Vitro Model | HCT 116 | Colon carcinoma | Homo sapiens (Human) | CVCL_0291 |
| RKO | Colon carcinoma | Homo sapiens (Human) | CVCL_0504 | |
| In Vivo Model | The concentration of HCT116 cells in the logarithmic phase was adjusted to 2.5×10⁷/mL, and the 200 μL cell suspension was inoculated subcutaneously on the right dorsal side of the mouse. When the average tumor volume reached 100 mm3, animals were randomized into 4 groups (5 mice for each group), control (Control, normal saline, 1 time per day, ip), irinotecan (IRI, 4 mg/kg irinotecan, 2 times per week, ip), EGCG (EGCG, 5 mg/kg, 1 time per day, ip), and irinotecan in combination with EGCG (IRI + EGCG). | |||
| Result | These reults confirmed that EGCG alone or in combination with irinotecan could up-regulate the GRP78, activate ERS of colorectal cancer cells, reduce intracellular reactive oxygen species and mitochondrial membrane potential, and induce apoptosis. The mouse xenograft experiment also confirmed the synergistic effect of EGCG and irinotecan on ERS and tumor cell.EGCG can induce GRP78-mediated endoplasmic reticulum stress and enhance the chemo-sensitivity of colorectal cancer cells when coadministered with irinotecan. | |||
| Pair Name | Epigallocatechin gallate, Irinotecan | |||
| Disease Info | [ICD-11: 2A00-2F9Z] | Solid tumour or cancer | Investigative | |
| Biological Phenomena | Inhibition-->P-gp expression | |||
| In Vivo Model | Adult, male Sprague–Dawley rats (300 ± 20 g body weight) were provided by the Laboratory Animal Center at National Yang-Ming University, the rats were initially anaesthetized with urethane 1 g/mL and α-chloralose 0.1 g/mL (1 mL/kg, i.p.), and remained anaesthetized throughout the experimental period. The femoral vein was exposed for further drug administration. | |||
| Result | EGCG was found to inhibit the transport of CPT-11 and SN-38 into the biliary elimination and their half-lives in plasma could be substantially prolonged. Based on the food-drug interaction, persons taking daily nutritional supplements should be warned of this interaction possibility. | |||
| No. | Title | Href |
|---|---|---|
| 1 | Food-drug interaction of (-)-epigallocatechin-3-gallate on the pharmacokinetics of irinotecan and the metabolite SN-38. Chem Biol Interact. 2008;174(3):177-182. doi:10.1016/j.cbi.2008.05.033 | Click |
| 2 | EGCG synergizes the therapeutic effect of irinotecan through enhanced DNA damage in human colorectal cancer cells. J Cell Mol Med. 2021 Aug;25(16):7913-7921. doi: 10.1111/jcmm.16718. | Click |
| 3 | EGCG Enhances the Chemosensitivity of Colorectal Cancer to Irinotecan through GRP78-MediatedEndoplasmic Reticulum Stress. J Oncol. 2022;2022:7099589. Published 2022 Sep 13. doi:10.1155/2022/7099589 | Click |
