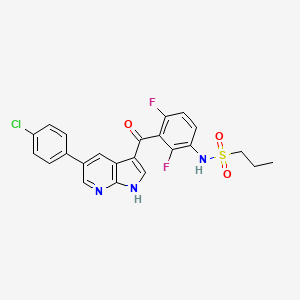
Vemurafenib, 918504-65-1, PLX4032, Zelboraf, 1029872-54-5, PLX-4032, N-(3-(5-(4-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluorophenyl)propane-1-sulfonamide, PLX 4032, RG7204, RG 7204, RO 5185426, Vemurafenib (PLX4032, RG7204), N-(3-{[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl}-2,4-difluorophenyl)propane-1-sulfonamide, vemurafenibum, RG-7204, RO5185426, UNII-207SMY3FQT, Vemurafenib (PLX4032), 207SMY3FQT, C23H18ClF2N3O3S, Ro 51-85426, CHEBI:63637, HSDB 8143, RO-5185426, N-[3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluorophenyl]propane-1-sulfonamide, RO-51-85426, DTXSID50238710, MFCD18074504, NSC761431, N-(3-((5-(4-Chlorophenyl)-1H-pyrrolo(2,3-b)pyridin-3-yl)carbonyl)-2,4- difluorophenyl)propane-1-sulfonamide, N-[3-[[5-(4-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-1-PropanesulfonaMide, Vemurafenib;PLX-4032, VEMURAFENIB (MART.), VEMURAFENIB [MART.], 1-Propanesulfonamide, N-(3-((5-(4-chlorophenyl)-1H-pyrrolo(2,3-b)pyridin-3- yl)carbonyl)-2,4-difluorophenyl)-, 1-PROPANESULFONAMIDE, N-[3-[[5-(4-CHLOROPHENYL)-1H-PYRROLO[2,3-B]PYRIDIN-3-YL]CARBONYL]-2,4-DIFLUOROPHENYL]-, N-{3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluorophenyl}propane-1-sulfonamide, Zelboraf (TN), 1-Propanesulfonamide, N-(3-((5-(4-chlorophenyl)-1H-pyrrolo(2,3-b)pyridin-3-yl)carbonyl)-2,4-difluorophenyl)-, n-(3-((5-(4-chlorophenyl)-1h-pyrrolo(2,3-b)pyridin-3-yl)carbonyl)-2,4-difluorophenyl)-1-propanesulfonamide, Vemurafenib [USAN], Vemurafenib [USAN:INN], N-(3-((5-(4-chlorophenyl)-1H-pyrrolo(2,3-b)pyridin-3-yl)carbonyl)-2,4-difluorophenyl)propane-1-sulfonamide, N-(3-(5-(4-Chlorophenyl)-1H-pyrrolo(2,3-b)pyridine-3-carbonyl)-2,4-difluorophenyl)propane-1-sulfonamide, N-(3-{(5-(4-chlorophenyl)-1H-pyrrolo(2,3-b)pyridin-3-yl)carbonyl}-2,4- difluorophenyl)propane-1-sulfonamide, 3og7, VEMURAFENIB [MI], 1415041-85-8, Vemurafenib; PLX4032, VEMURAFENIB [INN], VEMURAFENIB [JAN], VEMURAFENIB [VANDF], Vemurafenib (PLX4032)?, VEMURAFENIB [WHO-DD], SCHEMBL298931, Propane-1-sulfonic acid (3-, Vemurafenib (JAN/USAN/INN), GTPL5893, CHEMBL1229517, DTXCID00161201, EX-A053, L01XE15, VEMURAFENIB [ORANGE BOOK], GPXBXXGIAQBQNI-UHFFFAOYSA-N, HMS3265M03, HMS3265M04, HMS3265N03, HMS3265N04, HMS3654P09, HMS3748G15, BCP25783, EX-A1335, BDBM50396483, HB4285, NSC800964, s1267, AKOS007930804, AM81259, CCG-264883, CS-0216, DB08881, ME-0096, NSC-761431, NSC-800964, PB11741, NCGC00250399-01, NCGC00250399-05, NCGC00250399-08, AC-25010, HY-12057, N-[3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluoro-phenyl]propane-1-sulfonamide, SY067868, FT-0660388, FT-0675792, FT-0689782, NS00008103, SW218095-2, BRAF(V600E) Kinase Inhibitor RO5185426, A25476, A25742, BRAF (V600E) kinase inhibitor RO5185426, D09996, R-7204, AB01273970-01, AB01273970_03, EN300-23265091, Q423111, SR-01000941568, carbonyl]-2,4-difluorophenyl}propane-1-sulfonamide, J-522975, J-690009, SR-01000941568-1, BRD-K56343971-001-02-3, BRD-K56343971-001-05-6, PLX4032,Vemurafenib, RG7204, RO5185426, Zelboraf, N-{3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-, RO5185426 , RG7204 , PLX4032, [(2S,5R)-2,5-Dimethyl-4-[(tetrahydro-2H-pyran-4-yl)methyl]-1-piperazinyl][3-[(5-fluoro-2-methyl-4-pyrimidinyl)amino]-4,6-dihydro-6,6 -dimethylpyrrolo[3,4-c]pyrazol-5(1H)-yl]methanone, 1-Propanesulfonamide, N-(3-((5-(4-chlorophenyl)-1H- pyrrolo(2,3-b)pyridin-3- yl)carbonyl)-2,4-difluorophenyl)-, N-(3-(5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluorophenyl)-propane-1-sulfonamide, propane-1-sulfonic acid {3-[5-(4-chloro-phenyl)-1h-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide