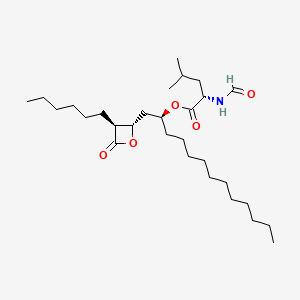
orlistat, 96829-58-2, Tetrahydrolipstatin, Xenical, Alli, Orlipastat, (-)-Tetrahydrolipstatin, Orlipastatum [INN-Latin], Ro-18-0647, Ro 18-0647/002, Orlistat (Alli, Xenical), (2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl (2S)-2-formamido-4-methylpentanoate, Orlistat (Standard), N-Formyl-L-leucine (1S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester, [(2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl] (2S)-2-formamido-4-methylpentanoate, L-Leucine, N-formyl-, (1S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester, MLS002207022, N-Formyl-L-leucine, ester with (3S,4S)-3-hexyl-4-((2S)-2-hydroxytridecyl)-2-oxetanone, CHEMBL175247, DTXSID8023395, MFCD05662360, 95M8R751W8, NSC-758881, Orlipastatum, SMR000466339, THLP, Ro-180647002, Ro-180647-002, Ro-18-0647/002, (S)-1-((2S,3S)-3-hexyl-4-oxooxetan-2-yl)tridecan-2-yl formyl-L-leucinate, L-Leucine, N-formyl-, (1S)-1-(((2S,3S)-3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl ester, tetrahydrolipastatin, C29H53NO5, DTXCID40820067, (S)-((S)-1-((2S,3S)-3-hexyl-4-oxooxetan-2-yl)tridecan-2-yl) 2-formamido-4-methylpentanoate, Xenical (TN), CAS-96829-58-2, SR-01000759417, orlistatum, Orlistat [USAN:INN:BAN], UNII-95M8R751W8, HSDB 7556, N-formyl-L-leucine (1S)-1-{[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl}dodecyl ester, NCGC00095128-01, (-)-Tetrahydrolipstatin; Orlistat; Ro 18-0647/002; Tetrahydrolipstatin; Xenical; L-Leucine, N-formyl-, 1-[(3-hexyl-4-oxo-2-oxetanyl)methyl]dodecyl ester, [2S-[2alpha(R*),3beta]]-, THL, KS-1183, Lipase Inhibitor, THL, ORLISTAT [HSDB], ORLISTAT [USAN], ORLISTAT [INN], ORLISTAT [JAN], ORLISTAT [MI], (-)-tetrahydrolipostatin, ORLISTAT [VANDF], R-212, ORLISTAT [MART.], ORLISTAT [USP-RS], ORLISTAT [WHO-DD], Orlistat (JAN/USP/INN), ORLISTAT [EMA EPAR], Orlistat, >=98%, solid, SCHEMBL16408, L-Leucine, N-formyl-, 1-((3-hexyl-4-oxo-2-oxetanyl)methyl)dodecyl ester, (2S-(2alpha(R*),3beta))-, MLS000759448, MLS001423955, BIDD:GT0853, ORLISTAT [ORANGE BOOK], GTPL5277, ORLISTAT [USP MONOGRAPH], BDBM24567, CHEBI:94686, HY-B0218R, AHLBNYSZXLDEJQ-FWEHEUNISA-N, Tetrahydrolipstatin;Ro-18-0647, HMS2051I08, HMS3413P06, HMS3677P06, HY-B0218, Tox21_111437, HB4009, s1629, AKOS015894875, Tox21_111437_1, BCP9001031, CCG-100851, DB01083, NC00101, NSC 758881, Ro18-0647, NCGC00165856-01, NCGC00165856-02, NCGC00165856-03, NCGC00165856-14, NCGC00165856-15, [(1S)-1-[[(2S,3S)-3-hexyl-4-oxo-oxetan-2-yl]methyl]dodecyl] (2S)-2-formamido-4-methyl-pentanoate, BO164179, R212, BCP0726000044, CS-0694775, NS00004788, O0381, SW197481-2, D04028, EN300-268136, AB00639987-09, AB00639987_10, Q424163, Q-201519, SR-01000759417-5, SR-01000759417-7, Z2379810072, Orlistat, United States Pharmacopeia (USP) Reference Standard, Orlistat, Pharmaceutical Secondary Standard; Certified Reference Material, (2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl N-formyl-L-leucinate, 2-formamido-3-[(3-hexyl-4-oxo-oxetan-2-yl)methyl]-2-isobutyl-tetradecanoate, N-formyl-L-leucine (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl ester, N-formyl-L-leucine-(S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]-dodecyl ester, (2S)-2-formamido-4-methylpentanoic acid [(2S)-1-[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]tridecan-2-yl] ester, [(2S)-1-[(2R,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl] (2R)-2-formamido-4-methylpentanoate, 104872-04-0, L-LEUCINE, N-FORMYL-, 1-((3-HEXYL-4-OXO-2-OXETANYL)METHYL)DODECYL ESTER, (2S-(2.ALPHA.(R*),3.BETA.))-