7,4'-dihydroxyflavanone, liquiritigenin



| Name | Liquiritigenin | ||
| PubChem CID | 114829 | ||
| Molecular Weight | 256.25g/mol | ||
| Synonyms |
7,4'-dihydroxyflavanone, liquiritigenin |
||
| Formula | C₁₅H₁₂O₄ | ||
| SMILES | C1C(OC2=C(C1=O)C=CC(=C2)O)C3=CC=C(C=C3)O | ||
| InChI | 1S/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1 | ||
| InChIKey | FURUXTVZLHCCNA-AWEZNQCLSA-N | ||
| CAS Number | 578-86-9 | ||
| ChEMBL ID | CHEMBL252642 | ||
| ChEBI ID | CHEBI:28777 | ||
| Herb ID | HBIN033376 | ||
| Drug Bank ID | DB03601 | ||
| KEGG ID | C09762 | ||
| Structure | 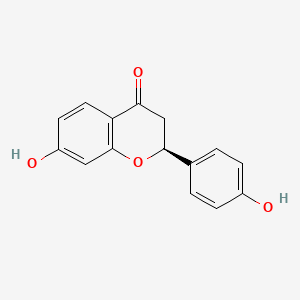
|
Download
2D
MOL
3D
MOL
|
|
| Chineses Pinyin | GanCao | ||
| Use Part | Root, Rhizome | ||
| Habitat | HeiLongJiang, JiLin, LiaoNing, NeiMengGu, GanSu, XinJiang | ||
| Flavor | Sweet | ||
| Meridian Tropism | Heart, Lung, Spleen, Stomach | ||
| Species |
>Kingdom: Viridiplantae
-->Phylum: Streptophyta
-->Class: Equisetopsida
-->Order: Fabales
-->Family: Fabaceae
-->Genus: Glycyrrhiza
-->Species: Glycyrrhiza uralensis
|
||
| Pair Name | Liquiritigenin, Cisplatin | |||
| Partner Name | Cisplatin | |||
| Disease Info | [ICD-11: 2C30] | Melanoma | Investigative | |
| Biological Phenomena | Inhibition-->Cell migration | |||
| Gene Regulation | Down-regulation | Expression | PIK3CA | hsa5290 |
| Down-regulation | Phosphorylation | AKT1 | hsa207 | |
| Down-regulation | Expression | MMP2 | hsa4313 | |
| Down-regulation | Expression | MMP9 | hsa4318 | |
| Up-regulation | Expression | PTEN | hsa5728 | |
| In Vitro Model | B16-F10 | Mouse melanoma | Mus musculus (Mouse) | CVCL_0159 |
| In Vivo Model | In vivo study demonstrated the enhancement effect of LQ on CDDP suppressing lung metastasis in a mice model being inoculated by the B16F10 melanoma cells. | |||
| Result | The results suggested that LQ plays an intensive role on CDDP suppressing invasion and metastasis through regulating the PI3 K/AKT signal pathway and suppressing the protein expression of MMP-2/9. | |||
| Pair Name | Liquiritigenin, [4-({6-[Allyl(methyl)amino]hexyl}oxy)-2-fluorophenyl](4-bromophenyl)methanone | |||
| Partner Name | [4-({6-[Allyl(methyl)amino]hexyl}oxy)-2-fluorophenyl](4-bromophenyl)methanone | |||
| Disease Info | [ICD-11: 2C60] | Breast cancer | Investigative | |
| Gene Regulation | Up-regulation | Expression | ESR2 | hsa2100 |
| Down-regulation | Expression | VEGFA | hsa7422 | |
| In Vitro Model | MCF-7 | Invasive breast carcinoma of no special type | Homo sapiens (Human) | CVCL_0031 |
| BT-474 | Invasive breast carcinoma of no special type | Homo sapiens (Human) | CVCL_0179 | |
| Result | The ERβ ligand LQ significantly enhanced the inhibition of breast-cancer cell viability and tumor-xenograft growth by RO. The anti-tumor properties of RO may in part be due to an off-target effect that reduces ERα and increases ERβ, the latter of which can then interact with LQ to promote anti-proliferative effects. The RO + LQ combination may have value when considering novel treatment strategies for hormone-dependent breast cancer. | |||
| No. | Title | Href |
|---|---|---|
| 1 | Liquiritigenin Potentiates the Inhibitory Effects of Cisplatin on Invasion and Metastasis Via Downregulation MMP-2/9 and PI3 K/AKT Signaling Pathway in B16F10 Melanoma Cells and Mice Model. Nutr Cancer. 2015;67(5):761-70. doi: 10.1080/01635581.2015.1037962. | Click |
| 2 | The estrogen receptor beta agonist liquiritigenin enhances the inhibitory effects of the cholesterol biosynthesis inhibitor RO 48-8071 on hormone-dependent breast-cancer growth. Breast Cancer Res Treat. 2022 Feb;192(1):53-63. doi: 10.1007/s10549-021-06487-y. | Click |
