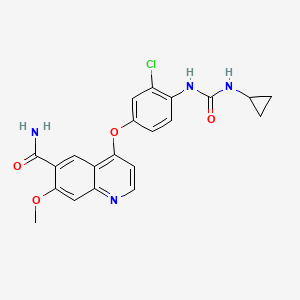
Lenvatinib, 417716-92-8, E7080, 4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxamide, E7080 (Lenvatinib), Lenvatinib (E7080), E-7080, ER-203492-00, E 7080, Lenvatinib free base, Kisplyx, UNII-EE083865G2, 4-{3-Chloro-4-[(Cyclopropylcarbamoyl)amino]phenoxy}-7-Methoxyquinoline-6-Carboxamide, 4-[3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide, CHEBI:85994, EE083865G2, 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxyquinoline-6-carboxamide, CHEMBL1289601, DTXSID50194605, 4-(3-chloro-4-(N'-cyclopropylureido)phenoxy)-7-methoxyquinoline-6-carboxamide, 417716-92-8 (free base), 4-(3-chloro-4-((cyclopropylaminocarbonyl)amino)phenoxy)-7-methoxy-6-quinolinecarboxamide, 4-[3-Chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide, 4-(3-CHLORO-4-((CYCLOPROPYLCARBAMOYL)AMINO)PHENOXY)-7-METHOXYQUINOLINE-6-CARBOXAMIDE, N-(4-((6-CARBAMOYL-7-METHOXYQUINOLIN-4-YL)OXY)-2-CHLOROPHENYL)-N'-CYCLOPROPYLUREA, 6-QUINOLINECARBOXAMIDE, 4-(3-CHLORO-4-(((CYCLOPROPYLAMINO)CARBONYL)AMINO)PHENOXY)-7-METHOXY-, Lenvatinib [USAN], Lenvatinib [USAN:INN], lenvatinibum, C21H19ClN4O4, 4-(3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide, 6-Quinolinecarboxamide, 4-[3-chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-; 4-[3-Chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide; E 7080; ER 203492-00; Lenvatinib; Lenvima, LEV, Lenvatinib; E7080, LENVATINIB [MI], Lenvatinib base- Bio-X, LENVATINIB [INN], Lenvatinib (USAN/INN), LENVATINIB [WHO-DD], MLS006011239, SCHEMBL864638, GTPL7426, Multi-Kinase Inhibitor E7080, AMY9240, DTXCID50117096, EX-A249, L01XE29, WOSKHXYHFSIKNG-UHFFFAOYSA-N, BCPP000247, HMS3244A07, HMS3244A08, HMS3244B07, HMS3654A14, BCP01799, BDBM50331094, MFCD16038644, NSC755980, NSC800781, s1164, AKOS025401742, BCP9000633, CCG-264842, CS-0109, DB09078, NSC-755980, NSC-800781, SB16580, NCGC00263198-01, NCGC00263198-04, NCGC00263198-07, AC-25047, AS-16203, BL164616, HY-10981, SMR004702999, DB-070219, FT-0700727, NS00069283, SW219259-1, D09919, EN300-7418350, A825653, J-513372, Q6523413, BRD-K39974922-001-02-7, Z2235801899, 4-[3-chloranyl-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carboxamide, 4-[3-chloro-4-[[(cyclopropylamino)-oxomethyl]amino]phenoxy]-7-methoxy-6-quinolinecarboxamide, 6-Quinolinecarboxamide, 4-(3-chloro-4- (((cyclopropylamino)carbonyl)amino)phenoxy)-7-methoxy-, 6-QUINOLINECARBOXAMIDE, 4-(3-CHLORO-4-(((CYCLOPROPYLAMINO)CARBONYL)AMINO)PHENOXY)- 7-METHOXY-