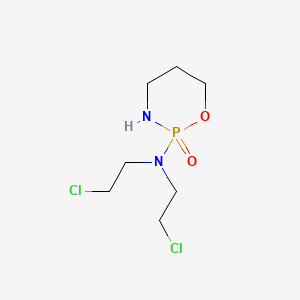
cyclophosphamide, 50-18-0, Cytoxan, Cyclophosphamid, Cyclophosphane, Endoxan, Cytophosphan, Procytox, Sendoxan, Clafen, Cyclophosphan, Cyclostin, Cyclophosphamidum, Cytophosphane, Endoxanal, Neosar, Claphene, Endoxana, Genoxal, Endoxan-Asta, Endoxan R, Zyklophosphamid, Mitoxan, Cyclophosphoramide, Cyklofosfamid, Endoxane, (RS)-Cyclophosphamide, Cyclophosphamide anhydrous, Cyclophosphanum, Enduxan, Semdoxan, Senduxan, (+-)-Cyclophosphamide, Ciclophosphamide, ASTA, Lyophilized Cytoxan, Ciclofosfamida, D,L-Cyclophosphamide, Rcra waste number U058, Asta B 518, (-)-Cyclophosphamide, Cycloblastin, NSC-26271, 2H-1,3,2-Oxazaphosphorin-2-amine, N,N-bis(2-chloroethyl)tetrahydro-, 2-oxide, Bis(2-chloroethyl)phosphoramide cyclic propanolamide ester, CB 4564, Cyclophosphamide (anhydrous), NCI-C04900, ASTA B518, SK 20501, NSC 26271, CHEBI:4027, B 518, Occupation, cyclophosphamide exposure, CCRIS 188, N,N-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide, 4-Hydroxy-cyclophosphan-mamophosphatide, HSDB 3047, Anhydrous cyclophosphamide, EINECS 200-015-4, Ciclofosfamide, N,N-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide, UNII-6UXW23996M, BRN 0011744, Cyclophosphamide-d4, AI3-26198, 6UXW23996M, Cyclophosphamide (INN), 1-Bis(2-chloroethyl)amino-1-oxo-2-aza-5-oxaphosphoridin, NSC26271, Cytoxan (TN), 2-(Bis(2-chloroethyl)amino)-2H-1,3,2-oxazaphosphorine 2-oxide, N,N-Bis(2-chloroethyl)-N',O-propylenephosphoric acid ester diamide, N,N-Di(2-chloroethyl)-N,O-propylene-phosphoric acid ester diamide, 50-18-0 (anhydrous), 2-[Bis(2-chloroethylamino)]-tetrahydro-2H-1,3,2-oxazaphosphorine-2-oxide, 2H-1,3,2-Oxazaphosphorin-2-amine, N,N-bis(2-chloroethyl)tetrahydro-,2-oxide, Phosphorodiamidic acid, N,N-bis(2-chloroethyl)-N'-(3-hydroxypropyl)-, intramol. ester, CY, DTXSID5020364, CYCLOPHOSPHAMIDE LYOPHILIZED, CYCLOPHOSPHAMIDE, ANHYDROUS, N,N-Bis(beta-chloroethyl)-N',O-propylenephosphoric acid ester diamide, 2-(Bis(2-chloroethyl)amino)tetrahydro-2H-1,3,2-oxazophosphorine 2-oxide, N,N-Bis(beta-chloroethyl)-N',O-trimethylenephosphoric acid ester diamide, Cyklofosfamid [Czech], N,N-bis(2-chloroethyl)-2-oxo-1,3,2$l^{5}-oxazaphosphinan-2-amine, N,N-Bis-(beta-chloraethyl)-N',O-propylen-phosphorsaeure-ester-diamid, Ciclophosphamide [INN], 2-[Bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide, 2H-1,3,2-Oxazaphosphorine, 2-(bis(2-chloroethyl)amino)tetrahydro-, 2-oxide, Zyklophosphamid [German], NSC-756711, Ciclophosphamide hydrat, Cytoxan (Lyophilized), CYCLOPHOSPHAMIDE [INN], Ciclofosfamida [INN-Spanish], Cyclofosphamide, Cyclophosphamidum [INN-Latin], UNII-8N3DW7272P, CYCLOPHOSPHAMIDE (IARC), CCRIS 7469, CYCLOPHOSPHAMIDE (USP-RS), Cyclophosphamide (anhydrous form), Cyclophosphamide (TN), CYCLOPHOSPHAMIDE (EP MONOGRAPH), CYCLOPHOSPHAMIDE (USP MONOGRAPH), SR-01000075737, NCGC00015209-05, RCRA waste no. U058, Cyclophosphamide [USP:INN], Cycloblastine, Cyclophospham, Cyclostine, Fosfaseron, Syklofosfamid, Carloxan, Cicloxal, Genuxal, Ledoxina, 2-(Bis(2-chloroethyl)amino)-2H-1,2-oxazaphosphorine 2-oxide, 2-[Bis(2-chloroethyl)amino]-2H-1,2-oxazaphosphorine 2-oxide, 2-(Bis(2-chloroethylamino))-tetrahydro-2H-1,3,2-oxazaphosphorine-2-oxide, (Bis(chloro-2-ethyl)amino)-2-tetrahydro-3,5,6-oxazaphosphorine-1,3,2-oxide-2 hydrate, [Bis(chloro-2-ethyl)amino]-2-tetrahydro-3,5,6-oxazaphosphorine-1,3,2-oxide-2 hydrate, 2-(BIS(2-CHLOROETHYL)AMINO)TETRAHYDRO-2H-1,3,2-OXAZAPHOSPHORINE 2-OXIDE, CYCLO-cell, N,N-Bis(beta-cloraethyl) N'-O-propylenphosphorildiamid monohydratum, 173547-45-0, bis(2-Chloroethyl)phosphamide cyclic propanolamide ester, CB-4564, Spectrum_000858, CHEMBL88, Spectrum2_001146, Spectrum3_000370, Spectrum4_000304, Spectrum5_000795, Lopac-C-0768, Epitope ID:131782, C 0768, N,N-Bis-(beta-chloraethyl)-N',O-propylen-phosphorsaeure-ester-diamid [German], SCHEMBL4346, (.+/-.)-Cyclophosphamide, Lopac0_000238, BSPBio_002099, KBioGR_000888, KBioSS_001338, DivK1c_000246, DTXCID70364, SPBio_001071, CYCLOPHOSPHAMIDE [HSDB], GTPL7154, SCHEMBL4345553, 2-H-1,3,2-Oxazaphosphorinane, SCHEMBL10262910, CMSMOCZEIVJLDB-UHFFFAOYSA-, KBio1_000246, KBio2_001338, KBio2_003906, KBio2_006474, KBio3_001319, CYCLOPHOSPAMIDE MONOHYDRATE, CYCLOPHOSPHAMIDE [WHO-DD], L01AA01, WLN: T6MPOTJ BO BN2G2G, NINDS_000246, 2-(bis(2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide, HMS2090A12, HMS3260P17, HMS3715J05, Pharmakon1600-01500213, AMY33449, BCP02139, Tox21_500238, BDBM50237604, N,N-bis(2-chloroethyl)-2-oxo-1,3,2lambda5-oxazaphosphinan-2-amine, NSC273033, NSC273034, NSC756711, STK177249, AKOS005410738, tetrahydro-N,N-bis(2-chloroethyl)-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide, CS-1425, CYCLOPHOSPHAMIDE ANHYDROUS [MI], DB00531, LP00238, NSC-273033, NSC-273034, SDCCGSBI-0050226.P004, IDI1_000246, N,3,2-oxazaphosphorin-2-amine-2-oxide, N,O-propylen-phosphorsaeure-ester-diamid, NCGC00015209-01, NCGC00015209-02, NCGC00015209-03, NCGC00015209-04, NCGC00015209-06, NCGC00015209-07, NCGC00015209-08, NCGC00015209-09, NCGC00015209-12, NCGC00015209-28, NCGC00091741-02, NCGC00091741-03, NCGC00260923-01, AS-73255, BP-25411, HY-17420, NCI60_002097, N,O-propylenephosphoric acid ester diamide, SBI-0050226.P003, DB-082057, N,O-propylene-phosphoric acid ester diamide, WR-138719, C2236, EU-0100238, FT-0624276, FT-0665387, N,O-trimethylenephosphoric acid ester diamide, NS00000339, EN300-74526, C07888, D07760, AB00053446-09, AB00053446-11, AB00053446-12, AB00053446_13, AB00053446_14, AB00053446_15, Q408524, W-60377, N,O-propylenephosphoric acid ester amide monohydrate, SR-01000075737-1, SR-01000075737-6, W-105248, BRD-A09722536-002-02-4, Z276509078, 2-(bis(2-chloroethyl)amino)-1,3,2-oxazaphosphinane2-oxide, Bis(2-chloroethyl)phosphoramide-cyclic propanolamide ester, 2-[bis(2-chloroethyl)amino]-1,3,2??-oxazaphosphinan-2-one, 1-BIS(2-CHLOROETHYL)AMINO-1-OXO-2-AZA-5-OXAPHOSPHORIDINE, 2-[bis(2-chloroethyl)amino]-1,3,2$l^{5}-oxazaphosphinan-2-one, 2-[bis(2-chloroethyl)amino]-1,3,2lambda5-oxazaphosphinan-2-one, N,N-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide #, 2-[Bis(2-chloroethyl)amino]tetrahydro-1,3,2-oxazaphosphorin-2-oxide, 2H-1,2-Oxazaphosphorine, 2-[bis(2-chloroethyl)amino]tetrahydro-, 2-oxide, 2H-1,3,2-Oxazaphosphorine, 2-(bis(2-chloroethyl)amino)tetrahydro-,2-oxide, N,N-Bis(.beta.-chloroethyl)-N',O-trimethylenephosphoric acid ester diamide, N,N-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine-2-oxide, N,N-Bis-(.beta.-chloraethyl)-N',O-propylen-phosphorsaeure-ester-diamid, N,N-DI(2-CHLOROETHYL)-N,O-PROPYLENE PHOSPHORIC ACID ESTER DIAMIDE, (+/-)-2-(BIS(2-CHLOROETHYL)AMINO)TETRAHYDRO-2H-1,3,2-OXAZAPHOSPHORINE 2-OXIDE, 2-(DI(2-CHLOROETHYLAMINO))-1-OXA-3-AZA-2-PHOSPHACYCLOHEXANE 2-OXIDE, 2-Bis(2-chloroethyl)amino)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide, 2H-1,2-Oxazaphosphorin-2-amine, N,N-bis(2-chloroethyl)tetrahydro-, 2-oxide, 2H-1,2-Oxazaphosphorin-2-amine, N,N-bis(2-chloroethyl)tetrahydro-, 2-oxide, (+)-, 2H-1,2-Oxazaphosphorin-2-amine, N,N-bis(2-chloroethyl)tetrahydro-, 2-oxide, (-)-, 2H-1,2-Oxazaphosphorine, 2-[bis(2-chloroethyl)amino]tetrahydro-, 2-oxide, monohydrate, 2H-1,3,2-oxazaphosphorin-2-amine, N,N-is(2-chloroethyl)tetrahydro-,2-oxide, N,N-bis(2-chloroethyl)-2-oxo-1-oxa-3-aza-2$l^{5}-phosphacyclohexan-2-amine, N,N-BIS(2-CHLOROETHYL)-N'-(3-HYDROXYPROPYL)PHOSPHORODIAMIDIC ACID INTRAMOL., N,N-BIS(BETA-CHLOROETHYL)N',O-TRIMETHYLENEPHOSPHORIC ACID ESTER DIAMIDE, Phosphorodiamidic acid,N-bis(2-chloroethyl)-N'-(3-hydroxypropyl)-, intramol. ester, InChI=1/C7H15Cl2N2O2P/c8-2-5-11(6-3-9)14(12)10-4-1-7-13-14/h1-7H2,(H,10,12)