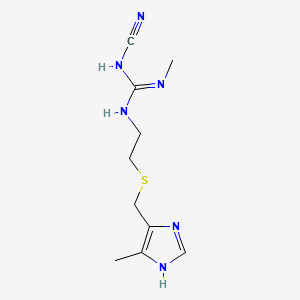
cimetidine, 51481-61-9, Tagamet, Ulcedin, Acinil, Dyspamet, Eureceptor, Gastromet, Tametin, Ulcimet, Cimal, Ulcomedina, Cimetag, Ulcedine, Tratul, Acibilin, Cimetum, Edalene, Ulcomet, Ulhys, Tagamet Hb, SKF-92334, Tagamet Hb 200, Cimetidina, Cimetidinum, Metracin, Valmagen, Brumetidina, Gastrobitan, Ulcestop, Evicer, Stomedine, Aciloc, 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine, N-Cyano-N'-methyl-N''-[2-[[(4-methyl-1H-imidazol-5-yl)methyl]thio]ethyl]guanidine, SKF 92334, Cimetidinum [INN-Latin], Cimetidina [INN-Spanish], DRG-0150, 2-Cyano-1-methyl-3-(2-(((5-methylimidazol-4-yl)methyl)thio)ethyl)guanidine, CCRIS 3247, CHEBI:3699, Topcare heartburn relief, NSC-335308, HSDB 3917, 1-Cyano-2-methyl-3-(2-(((5-methyl-4-imidazolyl)methyl)thio)ethyl)guanidine, FPF 1002, EINECS 257-232-2, UNII-80061L1WGD, N-Cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)guanidine, DTXSID4020329, 80061L1WGD, CHEMBL30, MFCD00133296, NSC 335308, Brumetadina, DTXCID40329, 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine, Guanidine, N-cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)-, MLS000069791, 2984-61-4, 76181-71-0, Ulcofalk, Peptol, 51481-61-9 (free), NSC335308, Guanidine, N''-cyano-N-methyl-N'-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)-, Guanidine, N-cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio) ethyl)-, Guanidine, N-cyano-N'-methyl-N''-[2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]ethyl]-, Guanidine, N-cyano-N'-Methyl-N''-[2-[[(5-Methyl-1H-iMidazol-4-yl)Methyl]thio]ethyl]-, (Z)-, N''-cyano-N-methyl-N'-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine, NSC 757428, 943920-67-0, Cimetidine [USAN:USP:INN:BAN:JAN], NCGC00015240-06, SMR000038895, Cimetidine 100 microg/mL in Acetonitrile, CIMETIDINE (IARC), CIMETIDINE [IARC], Cimetidinum (INN-Latin), Cimetidina (INN-Spanish), CIMETIDINE (MART.), CIMETIDINE [MART.], (Z)-3-cyano-1-methyl-2-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine, 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine, CIMETIDINE (USP-RS), CIMETIDINE [USP-RS], N-cyano-N'-methyl-N''-(2-([(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl)ethyl)guanidine, CIMETIDINE (EP MONOGRAPH), CIMETIDINE [EP MONOGRAPH], 4H-Dithieno[3,2-b :2',3'-d ]pyrrole, 4-(1-octylnonyl)-, CIMETIDINE (USP MONOGRAPH), CIMETIDINE [USP MONOGRAPH], Cimetidine (USAN:USP:INN:BAN:JAN), (E)-2-cyano-1-methyl-3-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)guanidine, (Z)-N''-cyano-N-methyl-N'-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine, 2-CYANO-1-METHYL-3-(2-(((5-METHYLIMIDAZOL-4-YL)METHYL)THIO)ETHYL)-GUANIDINE, Guanidine, N''-cyano-N-methyl-N'-[2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]ethyl]-, Tagamet (TN), Equaline acid reducer, SR-01000075260, SR-05000001434, cemitidine, metidine, Cimetidin, Equate Cimetidine, 1-cyano-2-methyl-3-(2-((5-methyl-1H-imidazol-4-yl)methylthio)ethyl)guanidine, 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylthio]ethyl]guanidine, 2-cyano-1-methyl-3-(2-(((5-methyl-1H-imidazol-4-yl)methyl)sulfanyl)ethyl)guanidine, 2-Cyano-1-methyl-3-(2-((5-methyl-1H-imidazol-4-yl)methylsulfanyl)ethyl)guanidine, 2-cyano-1-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine, Cimetidine,(S), N''-cyano-N-methyl-N'-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)guanidine, N-cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)sulfanyl)ethyl)guanidine, Prestwick_65, cimetidineacid reducer, TRATUE, N-Cyano-N'-Methyl-N''-(2-(((5-Methyl-1H-Imidazol-4-YL)Methyl)Thio)Ethyl) Guanidine, Cimetidine (Tagamet), Spectrum_000495, Tocris-0902, CIMETIDINE [MI], Opera_ID_314, SunMark heartburn relief, CIMETIDINE [INN], CIMETIDINE [JAN], Prestwick3_000026, Spectrum2_000782, Spectrum3_001389, Spectrum4_000812, Spectrum5_001541, CIMETIDINE [HSDB], CIMETIDINE [USAN], Lopac-C-4522, CIMETIDINE [VANDF], UPCMLD-DP029, 2-Cyano-1-methyl-3-[2-(5-methyl-1H-imidazol-4-yl-methylthio)ethyl]guanidine, C 4522, SCHEMBL1093, SCHEMBL1094, SKF-92334; Tagamet, Good Sense Heartburn Relief, CIMETIDINE [WHO-DD], CIMETIDINE [WHO-IP], Lopac0_000293, BSPBio_000091, BSPBio_002978, KBioGR_001323, KBioSS_000975, US9138393, Cimetidine, US9144538, Cimetidine, MLS001148596, MLS002153265, MLS002154178, DivK1c_000166, SPECTRUM1500684, SPBio_000884, BPBio1_000101, Cimetidine (JP17/USP/INN), GTPL1231, CIMETIDINE [ORANGE BOOK], SCHEMBL11282982, UPCMLD-DP029:001, BDBM22889, Cimetidine for system suitability, HMS500I08, KBio1_000166, KBio2_000975, KBio2_003543, KBio2_006111, KBio3_002198, A02BA01, Cimetidine for peak identification, NINDS_000166, BDBM181119, HMS1921C14, HMS2089O03, HMS2092I14, HMS2095E13, HMS2232F16, HMS3259M15, HMS3260L08, HMS3267A03, HMS3369L10, HMS3414I17, HMS3651E21, HMS3678I17, HMS3712E13, HMS3750I05, HMS3884I12, Pharmakon1600-01500684, Cimetidine 1.0 mg/ml in Methanol, CIMETIDINUM [WHO-IP LATIN], EX-A1088, SunMark heartburn reliefacid reducer, Tox21_110106, Tox21_201160, Tox21_500293, BDBM50103595, BDBM50403559, CCG-40160, NSC757428, s1845, STK528249, 1-Cyano-2-methyl-3-[2-[[(5-methylimidazol-4-yl)methyl]thio]ethyl]guanidine, AKOS005460997, AKOS015900557, AKOS015951369, AKOS016003398, AKOS016340377, AKOS026749950, AKOS032949548, AKOS040824773, Tox21_110106_1, AB03708, AC-8100, CCG-204388, CCG-220026, CCG-221597, DB00501, KS-5087, LP00293, NC00501, NSC-757428, SDCCGSBI-0050281.P005, 1-cyano-2-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine, Good Neighbor Pharmacy Heartburn Relief, IDI1_000166, N''-Cyano-N-methyl-N'-[2-[(5-methyl-1H-imidazol-4-yl)methylthio]ethyl]guanidine, N-Cyano-N'-methyl-[2-[[[5-methyl-1H-imidazol-4-yl]methyl]thio]ethyl]guanidine, NCGC00015240-01, NCGC00015240-02, NCGC00015240-03, NCGC00015240-04, NCGC00015240-05, NCGC00015240-07, NCGC00024859-01, NCGC00024859-02, NCGC00024859-03, NCGC00024859-04, NCGC00024859-05, NCGC00091439-01, NCGC00091439-02, NCGC00091439-03, NCGC00091439-04, NCGC00091439-05, NCGC00091439-07, NCGC00091439-10, NCGC00185989-01, NCGC00188961-01, NCGC00258712-01, NCGC00260978-01, 270574-63-5, HY-14289, NCI60_002936, SY057952, SBI-0050281.P004, DB-051971, EU-0100293, FT-0602955, NS00008665, SW196380-2, EN300-73705, C06952, D00295, F16651, AB00052157-03, AB00052157_04, AB00052157_05, A828616, EN300-27121291, EN300-27121293, L000186, L003827, L013434, Q409492, SR-05000001750, Q-200855, Q-200856, SR-01000075260-1, SR-01000075260-3, SR-05000001434-1, SR-05000001434-2, SR-05000001750-1, BRD-K18618618-001-01-6, BRD-K34157611-001-04-6, BRD-K34157611-001-07-9, Z1162463430, Cimetidine, European Pharmacopoeia (EP) Reference Standard, Cimetidine, United States Pharmacopeia (USP) Reference Standard, 1-Cyan-2-methyl-3-(2-{[(5-methylimidazol-4-yl)methyl]thio}ethyl)guanidin, Cimetidine, Pharmaceutical Secondary Standard; Certified Reference Material, N"-cyano-N-methyl-N'-[2-(5-methylimidazol-4-ylmethylthio)ethyl]-guanidine, N"-cyano-N-methyl-N'-[2-(5-methylimidazol-4-ylmethylthio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((4-methyl-5-imidazolyl)methylthio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((5-methyl-4-imidazolyl)methylthio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-(5-methyl-4-imidazolylmethylthio)ethyl]guanidine, (Z)-1-cyano-2-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine, 2-Chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1H-isoindol-1-yl)-benzenesulfonamide(Cimetidine), 2-Methyl-8-phenethyl-imidazo[1,2-a]pyridine-3-carboxylic acid methyl ester(cimetidine), 2-methylamino-2-[2-(4-methyl-1H-5-imidazolylmethylsulfanyl)ethylamino]-(E)-1-imino cyanide, 3-cyano-2-methyl-1-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine, 4-(((2-(((cyanoamino)(methylamino)methylene)amino)ethyl)thio)methyl)-5-methyl-1H-imidazole, Cimetidine for peak identification, European Pharmacopoeia (EP) Reference Standard, Cimetidine for system suitability, European Pharmacopoeia (EP) Reference Standard, Guanidine, N-cyano-N'-methyl-N''-[2-[[5-methyl-1H-imidazol-4-yl)methyl]thio]ethyl]-, N''-cyano-N-methyl-N'-(2-((5-methyl-1h-imidazol-4-yl)-methylthio)ethyl)guanidine, N''-cyano-N-methyl-N'-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine, N-cyano-N'-methyl-N"- [2-((5-methyl-4-imidazolyl)methylthio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((4-methyl-5-imidazolyl) methylthio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((4-methyl-5-imidazolyl)- methylthio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((4-methyl-5-imidazolyl)-methylthio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((4-methyl-5-imidazolyl)methyl-thio)ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((4-methyl-5-imidazolyl)methylthio)ethyl) guanidine, N-cyano-N'-methyl-N"-[2-((5-methyl-4-imidazolyl)methylthio)-ethyl]guanidine, N-cyano-N'-methyl-N"-[2-((5-methyl-4-imidazolyl)methylthio)ethyl]-guanidine, N-cyano-N'-methyl-N"-[2-{(5-methyl-1H-imidazol-4-yl)methylthio}ethyl]guanidine, N-CYANO-N'-METHYL-N''-(2(((5-METHYL-1H-IMIDAZOL-4-YL)METHYL)THIO)ETHYL), N-Cyano-N'-methyl-N''-(2-([(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl)ethyl)guanidine #, N-cyano-N'-methyl-N''-[2-[[(5-methylimidazol-4-yl]methyl]thio]ethyl)guanidine, N-cyano-N'-methyl-N-"-[2-((4-methyl-5-imidazolyl)methylthio)ethyl]guanidine, N-methyl-N'-{2-[(5-methylimidazol-4-yl)-methylthio]-ethyl}-N"-cyanoguanidine, N-methyl-N-[2-(5-methyl-1H-4-imidazolylmethylsulfanyl)ethyl]-1-cyanoiminomethanediamine, N-methyl-N-[2-(5-methyl-1H-4-imidazolylmethylsulfanyl)ethyl]imino(-N-cyano)methanediaminem, N-tert-Butyl-N''''''''-[4-(1H-imidazol-4-yl)-phenyl]-formamidine(cimetidine), (Cimetidine) N-Methyl-N''''''''-[2-(5-methyl-1H-imidazol-4-ylmethylsulfanyl)-ethyl]-guanidine,cyanide, (cimetidine) N-methyl-N-[2-(5-methyl-1H-4-imidazolylmethylsulfanyl)ethyl]cyanoiminomethanediamine, (Cimetidine)N-methyl-N-[2-(5-methyl-1H-4-imidazolylmethylsulfanyl)ethyl]cyanomethyliminomethanediamine, (E)-N''-CYANO-N-METHYL-N'-(2-{[(5-METHYL-1H-IMIDAZOL-4-YL)METHYL]SULFANYL}ETHYL)GUANIDINE, ; N''''''''''''''''-cyano-N-methyl-N''''''''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)-ethyl)guanidine, 2-methylamino-2-[2-(5-methyl-1H-4-imidazolylmethylsulfanyl)ethylamino]-(Z)-1-imino cyanide(cimetidine), N''''''''''''''''''''''''''''''''-cyano-N-methyl-N''''''''''''''''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine, N''''''''''''''''-cyano-N-methyl-N''''''''-({[(5-methyl-1H-imidazol-4-yl)methyl]thio}methyl)guanidine(cimetidine), N''''''''''''''''-cyano-N-methyl-N''''''''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]-lambda~4~-sulfanyl}ethyl)guanidine, N''''''''''''''''-cyano-N-methyl-N''''''''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine, N''''''''''''''''-cyano-N-methyl-N''''''''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine (Cimetidine), N''''''''''''''''-cyano-N-methyl-N''''''''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine(cimetidine), N-Cyano-N''''''''-methyl-N''''''''''''''''-(2-(((5-methyl-1H-imidazol-4-yl) methyl)thio)ethyl)guanidine(Cimetidine), N-cyanomethyl-N''''''''-methyl-N''''''''''''''''-[2-(5-methyl-1H-imidazol-4-ylmethylsulfanyl)-ethyl]-guanidine ( Cimetidine), N-Methyl-N''''''''-[2-(5-methyl-1H-imidazol-4-ylmethylsulfanyl)-ethyl]-cyanoguanidine(cimetidine), N-Methyl-N''''''''-[2-(5-methyl-1H-imidazol-4-ylmethylsulfanyl)-ethyl]-N''''''''''''''''-Cyano-guanidine, N-Methyl-N''''''''-[2-(5-methyl-1H-imidazol-4-ylmethylsulfanyl)-ethyl]-N''''''''''''''''-cyano-guanidine(Cimetidine), N-methyl-N''''''''-cyano-N''''''''''''''''-[2-(5-methyl-1H-imidazol-4-ylmethylsulfanyl)-ethyl]-guanidine, N-methyl-N-[2-(5-methyl-1H-4-imidazolylmethylsulfanyl)ethyl]cyanoiminomethanediamine (cimetidine)