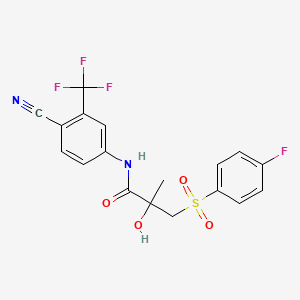
bicalutamide, 90357-06-5, Casodex, Cosudex, Calutide, ICI 176334, Bicalutamide (CDX), ICI-176334, N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methylpropanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-hydroxy-2-methylpropanamide, ICI 176,334, A0Z3NAU9DP, NSC-759816, DTXSID2022678, C18H14F4N2O4S, CHEMBL409, ICI176,334-1, DTXCID00209197, MFCD00869971, NSC722665, N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide, Kalumid, BICALUTAMIDE (MART.), BICALUTAMIDE [MART.], Propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-, BICALUTAMIDE (USP-RS), BICALUTAMIDE [USP-RS], Raffolutil, BICALUTAMIDE (EP MONOGRAPH), BICALUTAMIDE (USP IMPURITY), BICALUTAMIDE [EP MONOGRAPH], BICALUTAMIDE [USP IMPURITY], BICALUTAMIDE (USP MONOGRAPH), BICALUTAMIDE [USP MONOGRAPH], PROPANAMIDE, N-(4-CYANO-3-(TRIFLUOROMETHYL)PHENYL)-3-((4-FLUOROPHENYL)SULFONYL)-2-HYDROXY-2-METHYL-, (+/-)-, SMR000466329, Casodex (TN), CAS-90357-06-5, SR-01000759410, UNII-A0Z3NAU9DP, CHEBI:3090, bicalutamida, bicalutamidum, BRN 5364666, Bicadex, Bicalox, Bicamide, Bicatlon, Bicusan, Binabic, Calumid, Calutol, Ormandyl, Bical, Bypro, Bicalutamide (JAN/USP/INN), Propanamide,, CCRIS 8728, HSDB 7655, NCGC00167487-01, (RS)-bicalutamide, racemic bicalutamide, Bicalutamide [USAN:USP:INN:BAN], (+-)-bicalutamide, (+-)-4'-Cyano-alpha,alpha,alpha-trifluoro-3-((p-fluorophenyl)sulfonyl)-2-methyl-m-lactotoluidide, KS-1161, Bicalutamide (Casodex), CPD000466329, BICALUTAMIDE [MI], BICALUTAMIDE [INN], BICALUTAMIDE [JAN], BICALUTAMIDE [HSDB], BICALUTAMIDE [USAN], SCHEMBL3611, 4'-cyano-3-(4-fluorophenylsulfonyl)-2-hydroxy-2-methyl-3'-(trifluoromethyl)propionanilide, BICALUTAMIDE [VANDF], -3-(4-fluorophenylsulfonyl), MLS000759437, MLS001424047, Propanamide, N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methyl-, (+-)-, BICALUTAMIDE [WHO-DD], -2-hydroxy-2-methylpropanamide, GTPL2863, BDBM18525, Bicalutamide Crystalline Form II, CHEBI:91617, EX-A962, L02BB03, CHEBI:144093, BCPP000337, BICALUTAMIDE [ORANGE BOOK], DTXSID501016794, HMS2051B13, HMS2089N12, HMS2232H03, HMS3263M13, HMS3372K05, HMS3393B13, HMS3654K18, HMS3714P13, Pharmakon1600-01504827, AMY33430, BCP02110, Tox21_112488, Tox21_303560, Tox21_501026, NSC759816, s1190, AKOS015895073, AC-4232, BCP9000408, CCG-100951, CCG-220876, CCG-222330, CS-1296, DB01128, LP01026, NC00201, NSC 759816, NSC-722665, SB17301, SDCCGSBI-0633779.P001, 4-cyano-3-trifluoromethyl-N-(3-p-fluorophenylsulfonyl-2-hydroxy-2-methylpropionyl)aniline, N-(4-cyano-3-(trifluoromethyl)phenyl), NCGC00167977-01, NCGC00167977-02, NCGC00167977-03, NCGC00167977-09, NCGC00167977-20, NCGC00257459-01, NCGC00261711-01, HY-14249, DB-041165, B3206, FT-0618286, FT-0631069, FT-0663100, NS00000400, SW197581-4, Bicalutamide (CDX), >=98% (HPLC), powder, C08160, D00961, inverted exclamation markY98% (HPLC), powder, AB00639963-06, AB00639963-08, AB00639963-09, AB00639963_10, EN300-1715981, A803039, A843528, Q1988832, SR-01000759410-4, SR-01000759410-5, BRD-A29485665-001-03-7, Z2108698963, Bicalutamide, British Pharmacopoeia (BP) Reference Standard, Bicalutamide, European Pharmacopoeia (EP) Reference Standard, Bicalutamide, United States Pharmacopeia (USP) Reference Standard, 4'-cyano-3-[(4- fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-3'-trifluoromethylpropionanilide, 4'-cyano-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-3'-trifluoromethylpropionanilide, 4'-Cyano-alpha,alpha,alpha-trifuloro-3-((p-fluorophenyl)sulfonyl)-2-methyl-m-lactotoluidide, Bicalutamide for system suitability, European Pharmacopoeia (EP) Reference Standard, Bicalutamide, Pharmaceutical Secondary Standard; Certified Reference Material, (+-)-4'-Cyano-alpha,alpha,alpha-trifluoro-3-((p- fluorophenyl)sulfonyl)-2-methyl-m-lactotoluidide, (+/-)-4'-CYANO-.ALPHA.,.ALPHA.,.ALPHA.-TRIFLUORO-3-((P-FLUOROPHENYL)SULFONYL)-2-METHYL-M-LACTOTOLUIDIDE, (+/-)-N-(4-Cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methylpropanamide, (RS)-4'-cyano-alpha',alpha',alpha',-trifluoro-3-(4-fluorophenylsulphonyl)-2-hydroxy-2-methylpropiono-m-toluidide, N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methyl-N-phenylpropanamide, N-(4-cyano-3-(trifluoromethyl)phenyl)-3-(4-fluorophenylsulfonyl)-2-hydroxy-2-methylpropanamide, N-(4-Cyano-3-(trifluoromethyl)phenyl)3-3((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methyl-propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorobenzenesulfonyl)-2-hydroxy-2-methylpropanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenyl)sulfonyl-2-methyl-2-oxidanyl-propanamide, N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorobenzene)sulfonyl]-2-hydroxy-2-methylpropanamide, N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropionamide, N-[4-cyano-3-trifluoromethyl-phenyl]-3-[4-fluorophenyl-sulfonyl]-2-hydroxy-2-methyl-propionamide, rac-N-(4-cyano-3-(trifluoromethyl)phenyl)-3-((4-fluorophenyl)sulfonyl)-2-hydroxy-2-methylpropanamide