NPs Basic Information
|
Name |
Bergapten
|
| Molecular Formula | C12H8O4 | |
| IUPAC Name* |
4-methoxyfuro[3,2-g]chromen-7-one
|
|
| SMILES |
COC1=C2C=CC(=O)OC2=CC3=C1C=CO3
|
|
| InChI |
InChI=1S/C12H8O4/c1-14-12-7-2-3-11(13)16-10(7)6-9-8(12)4-5-15-9/h2-6H,1H3
|
|
| InChIKey |
BGEBZHIAGXMEMV-UHFFFAOYSA-N
|
|
| Synonyms |
Bergapten; 5-Methoxypsoralen; 484-20-8; bergaptene; Heraclin; Majudin; 4-Methoxy-7H-furo[3,2-g]chromen-7-one; BERGAPTAN; Psoraderm; 5-Mop; 5-Methoxy psoralen; O-Methylbergaptol; Geralen; 5-Methoxy-6,7-furanocoumarin; 5-Methoxyfuranocoumarin; 4-methoxyfuro[3,2-g]chromen-7-one; Pentaderm; 7H-Furo[3,2-g][1]benzopyran-7-one, 4-methoxy-; 4-Methoxy-7H-furo(3,2-g)(1)benzopyran-7-one; 4-Methoxyfuro[3,2-g]benzopyran-7-one; 5-methoxypsoralene; NSC 95437; Bergaptene (DCF); 4-methoxy-7H-furo[3,2-g][1]benzopyran-7-one; 5-Methoxypsoralen with ultraviolet A therapy; NSC95437; 6-Hydroxy-4-methoxy-5-benzofuranacrylic acid, gamma-lactone; NSC-95437; 4-Methoxy-furo[3,2-g]chromen-7-one; 7H-Furo(3,2-g)(1)benzopyran-7-one, 4-methoxy-; 4-Methoxyfuro[3,2-g]benzopyrane-7-one; CHEMBL24171; 4FVK84C92X; DSSTox_CID_5560; DSSTox_RID_77830; DSSTox_GSID_25560; 5 methoxypsoralen; CAS-484-20-8; SMR000112435; CCRIS 4348; HSDB 3466; SR-05000002173; EINECS 207-604-5; BRN 0019560; UNII-4FVK84C92X; Pentaderm (TN); 5-methoxy-psoralen; MFCD00010272; 5-methoxyfurano[3,2-g]chromen-2-one; Spectrum_000794; 5-methoxy-2H-furo[3,2-g]chromen-2-one; BERGAPTEN [MI]; Spectrum2_000534; Spectrum3_000663; Spectrum4_001478; Spectrum5_000155; 5-Methoxypsoralen, 99%; 5-Methoxypsoralen;Heraclin; bmse000758; 5-Methoxypsoralen (obsol.); 7H-Furo[3, 4-methoxy-; Oprea1_562364; SCHEMBL50066; BSPBio_002325; KBioGR_002055; KBioSS_001274; SPECTRUM300546; 5-19-06-00004 (Beilstein Handbook Reference); MLS002207272; MLS002454380; Bergapten, analytical standard; DivK1c_000529; SPBio_000547; MEGxp0_000990; DTXSID1025560; ACon0_000984; ACon1_001979; CHEBI:18293; HMS501K11; KBio1_000529; KBio2_001274; KBio2_003842; KBio2_006410; KBio3_001545; ZINC57731; 5-METHOXYPSORALEN [IARC]; NINDS_000529; HMS1923G13; HMS2268M24; HMS3652F19; Pharmakon1600-00300546; 5-METHOXYPSORALEN [MART.]; 5-METHOXYPSORALEN [WHO-DD]; BCP30865; HY-N0370; TNP00299; Tox21_202357; Tox21_303255; BDBM50067880; CCG-39946; NSC755877; s4239; STK333038; AKOS000276715; DB12216; DS-2970; NSC-755877; SDCCGMLS-0066492.P001; IDI1_000529; NCGC00017357-01; NCGC00017357-02; NCGC00017357-03; NCGC00017357-04; NCGC00017357-05; NCGC00017357-06; NCGC00017357-07; NCGC00017357-08; NCGC00091582-01; NCGC00091582-02; NCGC00091582-03; NCGC00091582-04; NCGC00178705-01; NCGC00178705-02; NCGC00256998-01; NCGC00259906-01; AC-20189; AC-34208; NCI60_042121; SBI-0051583.P002; DB-051552; 4-Methoxy-7H-furo[3,2-g]benzopyran-7-one; B2840; FT-0603416; SW220008-1; 4-Methoxy-7H-furo[3,2-g]chromen-7-one #; C01557; D07521; AB00052148_06; AB00052148_07; 484B208; A827532; Q414779; Q-100536; SR-05000002173-2; SR-05000002173-3; SR-05000002173-5; BRD-K12968785-001-02-6; BRD-K12968785-001-03-4; BRD-K12968785-001-06-7; BRD-K12968785-001-11-7; 6-Hydroxy-4-methoxy-5-benzofuranacrylic acid, .gamma.-lactone
|
|
| CAS | 484-20-8 | |
| PubChem CID | 2355 | |
| ChEMBL ID | CHEMBL24171 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 216.19 | ALogp: | 2.3 |
| HBD: | 0 | HBA: | 4 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 48.7 | Aromatic Rings: | 3 |
| Heavy Atoms: | 16 | QED Weighted: | 0.585 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.783 | MDCK Permeability: | 0.00002820 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0.931 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.117 |
| 30% Bioavailability (F30%): | 0.989 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.034 | Plasma Protein Binding (PPB): | 85.24% |
| Volume Distribution (VD): | 0.618 | Fu: | 14.27% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.988 | CYP1A2-substrate: | 0.95 |
| CYP2C19-inhibitor: | 0.704 | CYP2C19-substrate: | 0.121 |
| CYP2C9-inhibitor: | 0.127 | CYP2C9-substrate: | 0.865 |
| CYP2D6-inhibitor: | 0.793 | CYP2D6-substrate: | 0.875 |
| CYP3A4-inhibitor: | 0.592 | CYP3A4-substrate: | 0.328 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.128 | Half-life (T1/2): | 0.462 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.251 | Human Hepatotoxicity (H-HT): | 0.31 |
| Drug-inuced Liver Injury (DILI): | 0.887 | AMES Toxicity: | 0.061 |
| Rat Oral Acute Toxicity: | 0.386 | Maximum Recommended Daily Dose: | 0.245 |
| Skin Sensitization: | 0.27 | Carcinogencity: | 0.863 |
| Eye Corrosion: | 0.007 | Eye Irritation: | 0.922 |
| Respiratory Toxicity: | 0.627 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001537 |  |
0.417 | D08SKH |  |
0.533 | ||
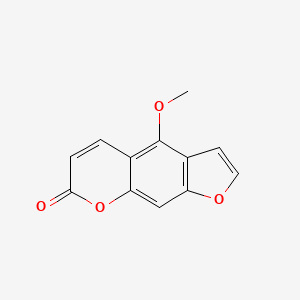
| ENC002586 | 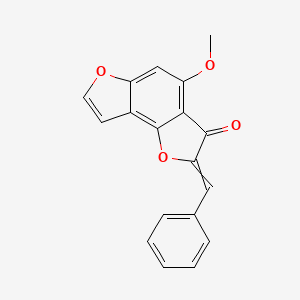 |
0.392 | D0G4KG | 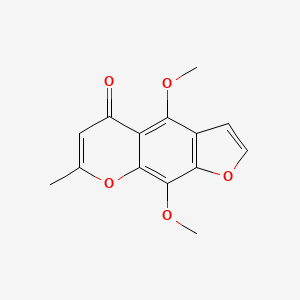 |
0.375 | ||
| ENC001539 |  |
0.390 | D0R2OA |  |
0.309 | ||
| ENC000798 |  |
0.390 | D06GCK | 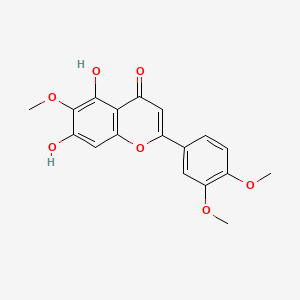 |
0.278 | ||
| ENC001623 | 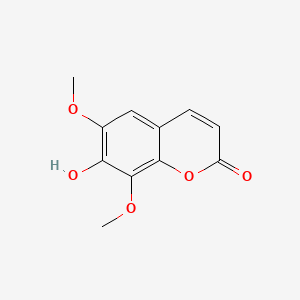 |
0.385 | D0IT2X | 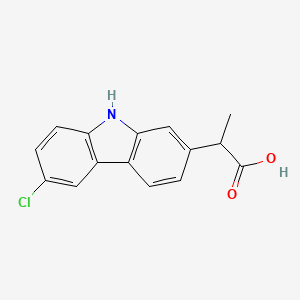 |
0.253 | ||
| ENC001562 | 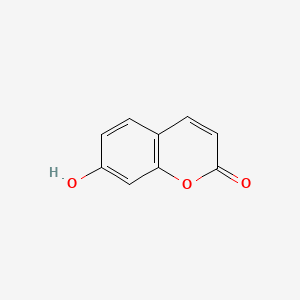 |
0.379 | D09WKB | 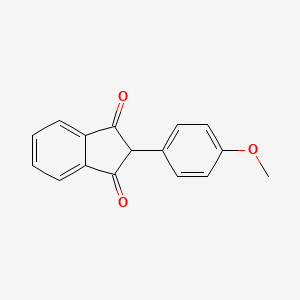 |
0.250 | ||
| ENC001524 | 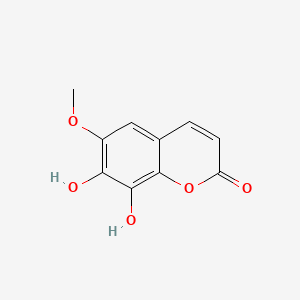 |
0.359 | D08CCE |  |
0.247 | ||
| ENC001515 | 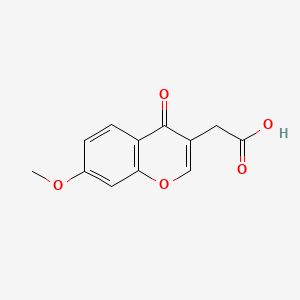 |
0.348 | D0QV5T |  |
0.247 | ||
| ENC000025 |  |
0.345 | D0G5UB | 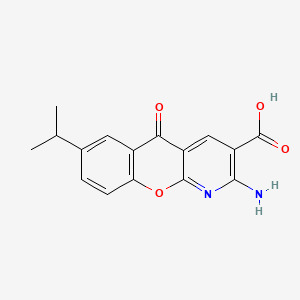 |
0.247 | ||
| ENC001561 | 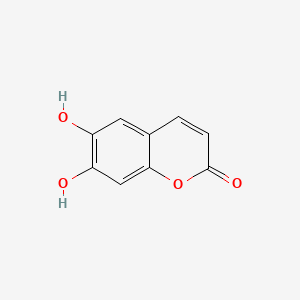 |
0.344 | D02TJS | 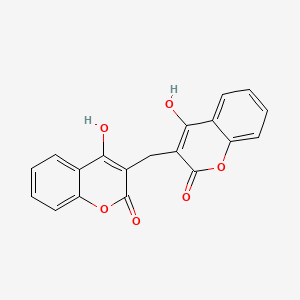 |
0.245 | ||