NPs Basic Information

|
Name |
Coumarin
|
| Molecular Formula | C9H6O2 | |
| IUPAC Name* |
chromen-2-one
|
|
| SMILES |
C1=CC=C2C(=C1)C=CC(=O)O2
|
|
| InChI |
InChI=1S/C9H6O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H
|
|
| InChIKey |
ZYGHJZDHTFUPRJ-UHFFFAOYSA-N
|
|
| Synonyms |
coumarin; 91-64-5; 2H-Chromen-2-one; 2H-1-Benzopyran-2-one; 1,2-Benzopyrone; cumarin; chromen-2-one; Rattex; Tonka bean camphor; Coumarinic anhydride; Coumarine; cis-o-Coumarinic acid lactone; o-Hydroxycinnamic acid lactone; Coumarinic lactone; Benzo-alpha-pyrone; o-Hydroxycinnamic lactone; 2-Oxo-1,2-benzopyran; Kumarin; 2H-Benzo(b)pyran-2-one; Benzo-a-pyrone; Kumarin [Czech]; 2H-1-Benzopyran, 2-oxo-; 5,6-Benzo-2-pyrone; 5,6-Benzo-alpha-pyrone; 2H-Benzo[b]pyran-2-one; Caswell No. 259C; o-Coumaric acid lactone; o-Hydroxyzimtsaure-lacton; NCI C07103; cis-o-Coumaric acid anhydride; chromenone; Coumarinum; 103802-83-1; o-Hydroxyzimtsaure-lacton [German]; NSC 8774; 2-Propenoic acid, 3-(2-hydroxyphenyl)-, delta-lactone; EPA Pesticide Chemical Code 127301; BRN 0383644; Benzo-.alpha.-pyrone; Cinnamic acid, o-hydroxy-, delta-lactone; CHEBI:28794; AI3-00753; NSC-8774; o-hydroxycinnamic acid delta-lactone; 3-(2-Hydroxyphenyl)-2-propenoic delta-lactone; A4VZ22K1WT; CHEMBL6466; Nci-c07103; 2-Propenoic acid, 3-(2-hydroxyphenyl)-delta-lactone; MLS000028741; DTXSID7020348; NSC8774; NCGC00091502-01; Coumarin, >=98%; SMR000059040; 1-Benzopyran-2-one; DSSTox_CID_348; 2h-chromene-2-one; DSSTox_RID_75529; DSSTox_GSID_20348; 2-Propenoic acid, 3-(2-hydroxyphenyl)-, d-lactone; 2-Propenoic acid, 3-(2-hydroxyphenyl)-, .delta.-lactone; benzopyranone; CAS-91-64-5; Coumarin [NF]; COU; CCRIS 181; 2H-Benzopyran-2-one; HSDB 1623; SR-01000721887; 2-oxo-2H-1-benzopyran; EINECS 202-086-7; MFCD00006850; UNII-A4VZ22K1WT; chromen-one; a coumarin; coumarin-; d-lactone; alpha-benzopyrone; benzopyrylium olate; Coumarin (DCF); 1, 2-Benzopyrone; a 1,2-benzopyrone; Venalot mono (TN); Coumarin (prohibited); Spectrum_001336; ST023509; COUMARIN [HSDB]; COUMARIN [IARC]; COUMARIN [INCI]; Opera_ID_268; 2H-Chromen-2-one #; 3-(2-hydroxyphenyl)-; COUMARIN [MI]; 2H-1-benzopyran-2-on; Spectrum2_000303; Spectrum3_001772; Spectrum4_001818; Spectrum5_000555; COUMARINUM [HPUS]; COUMARIN [MART.]; bmse000077; COUMARIN [WHO-DD]; Epitope ID:114082; EC 202-086-7; SCHEMBL6252; WLN: T66 BOVJ; BSPBio_003263; KBioGR_002460; KBioSS_001816; 5-17-10-00143 (Beilstein Handbook Reference); MLS001148422; MLS002454395; {2H-Benzo[b]pyran-2-one}; BIDD:ER0667; SPECTRUM1400208; SPBio_000266; Cinnamic acid, .delta.-lactone; Coumarin, >=99% (HPLC); BDBM12342; KBio2_001816; KBio2_004384; KBio2_006952; KBio3_002764; ZINC74709; GLXC-19130; HMS1923M11; HMS2091E19; HMS2232H18; HMS3369L08; HMS3652B05; HMS3885D09; Pharmakon1600-01400208; AMY37188; HY-N0709; Tox21_111141; Tox21_202427; Tox21_300057; CCG-38580; NSC755852; s4170; STK066167; COUMARIN (PROHIBITED) [FHFI]; AKOS000120175; Tox21_111141_1; 2H-chromen-2-one (ACD/Name 4.0); CR-0048; CS-8148; DB04665; NSC-755852; SDCCGMLS-0066912.P001; NCGC00091502-02; NCGC00091502-03; NCGC00091502-04; NCGC00091502-05; NCGC00091502-06; NCGC00091502-07; NCGC00091502-08; NCGC00091502-09; NCGC00091502-11; NCGC00091502-16; NCGC00254092-01; NCGC00259976-01; Coumarin 1000 microg/mL in Acetonitrile; NCI60_041938; SBI-0061760.P002; DB-057267; Cinnamic acid, o-hydroxy-, .delta.-lactone; Coumarin, Vetec(TM) reagent grade, >=99%; FT-0606287; FT-0665197; SW220278-1; EN300-18115; A14543; BIM-0061760.0001; C05851; D07751; D81844; AB00375898_11; AB00375898_12; Coumarin, primary pharmaceutical reference standard; Q111812; CU-01000013121-2; Q-100890; SR-01000721887-2; SR-01000721887-3; BRD-K23913458-001-02-5; BRD-K23913458-001-13-2; Coumarin, certified reference material, TraceCERT(R); Z57169486; Coumarin, European Pharmacopoeia (EP) Reference Standard; F3096-1712; COUMARIN (CONSTITUENT OF CINNAMOMUM CASSIA BARK) [DSC]; COUMARIN (CONSTITUENT OF CINNAMOMUM VERUM BARK) [DSC]; D3E956C4-9541-4F57-9435-7D915C38E19E; 2h-1-benzopyran-2-one;coumarin;2h-chromen-2-one;coumarin ;coumarin (2h-1-benzopyran-2-one) (chromen-2-one);2h-1-benzopyran-2-one coumarin 2h-chromen-2-one coumarin coumarin (2h-1-benzopyran-2-one) (chromen-2-one)
|
|
| CAS | 91-64-5 | |
| PubChem CID | 323 | |
| ChEMBL ID | CHEMBL6466 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 146.14 | ALogp: | 1.4 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 11 | QED Weighted: | 0.534 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.582 | MDCK Permeability: | 0.00002110 |
| Pgp-inhibitor: | 0.01 | Pgp-substrate: | 0.642 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.993 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.172 | Plasma Protein Binding (PPB): | 87.26% |
| Volume Distribution (VD): | 0.833 | Fu: | 12.25% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.984 | CYP1A2-substrate: | 0.904 |
| CYP2C19-inhibitor: | 0.564 | CYP2C19-substrate: | 0.111 |
| CYP2C9-inhibitor: | 0.081 | CYP2C9-substrate: | 0.635 |
| CYP2D6-inhibitor: | 0.212 | CYP2D6-substrate: | 0.638 |
| CYP3A4-inhibitor: | 0.029 | CYP3A4-substrate: | 0.296 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.851 | Half-life (T1/2): | 0.721 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.165 | Human Hepatotoxicity (H-HT): | 0.102 |
| Drug-inuced Liver Injury (DILI): | 0.845 | AMES Toxicity: | 0.219 |
| Rat Oral Acute Toxicity: | 0.672 | Maximum Recommended Daily Dose: | 0.023 |
| Skin Sensitization: | 0.241 | Carcinogencity: | 0.892 |
| Eye Corrosion: | 0.77 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.115 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001562 | 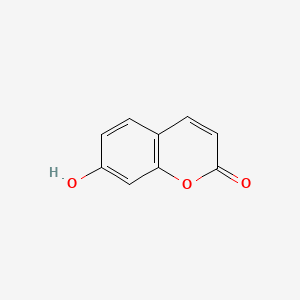 |
0.571 | D02WCI |  |
0.358 | ||
| ENC002806 | 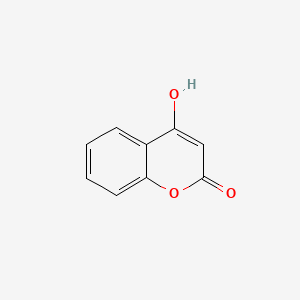 |
0.500 | D08SKH |  |
0.345 | ||
| ENC000675 |  |
0.488 | D0QV5T |  |
0.333 | ||
| ENC002764 | 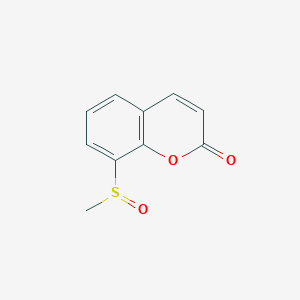 |
0.479 | D0D5GG | 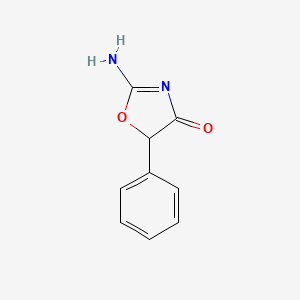 |
0.327 | ||
| ENC000169 |  |
0.455 | D0B1FE |  |
0.317 | ||
| ENC000714 |  |
0.426 | D09ZIS |  |
0.315 | ||
| ENC000167 |  |
0.422 | D0K1XK |  |
0.314 | ||
| ENC000036 |  |
0.420 | D0O6IZ |  |
0.311 | ||
| ENC001561 |  |
0.417 | D0E3OF |  |
0.311 | ||
| ENC000047 |  |
0.409 | D03GET |  |
0.308 | ||