NPs Basic Information

|
Name |
Hymecromone
|
| Molecular Formula | C10H8O3 | |
| IUPAC Name* |
7-hydroxy-4-methylchromen-2-one
|
|
| SMILES |
CC1=CC(=O)OC2=C1C=CC(=C2)O
|
|
| InChI |
InChI=1S/C10H8O3/c1-6-4-10(12)13-9-5-7(11)2-3-8(6)9/h2-5,11H,1H3
|
|
| InChIKey |
HSHNITRMYYLLCV-UHFFFAOYSA-N
|
|
| Synonyms |
4-methylumbelliferone; 90-33-5; Hymecromone; 7-HYDROXY-4-METHYLCOUMARIN; 7-Hydroxy-4-methyl-2H-chromen-2-one; Imecromone; beta-Methylumbelliferone; Cantabiline; Cholestil; Mendiaxon; 4-Methyl-7-hydroxycoumarin; Bilcolic; Bilicante; Cantabilin; 4-MU; Coumarin 4; Cholonerton; Hymecromon; Crodimon; Eurogale; Medilla; Cumarote-C; Omega 127; Pilot 447; 2H-1-Benzopyran-2-one, 7-hydroxy-4-methyl-; 4-Methylumbelliferon; Himecromona; Hymecromonum; 7-Hydroxy-4-methyl-2H-1-benzopyran-2-one; NSC 9408; NSC 19026; 7-Hydroxy-4-methyl-2-oxo-2H-1-benzopyran; 7-hydroxy-4-methylchromen-2-one; LM 94; Coumarin, 7-hydroxy-4-methyl-; LM-94; 7-hydroxy-4-methyl-chromen-2-one; MFCD00006866; 7-hydroxy-4-methyl-coumarin; 2H-1-Benzopyran, 7-hydroxy-4-methyl-2-oxo-; 2H-1-Benzopyren-2-one, 7-hydroxy-4-methyl-; 7-Hydroxy-4-methyl-2-oxo-3-chromene; 4-Methylumbelliferone (4-MU); NSC9408; NSC-9408; NSC19026; NSC-19026; .beta.-Methylumbelliferone; 7-hydroxy-4-methyl coumarin; 4-Methyl-7-hydroxy-coumarin; CHEMBL12208; Resocyanine; CHEBI:17224; 3T5NG4Q468; CAS-90-33-5; NCGC00016345-01; DSSTox_CID_5670; DSSTox_RID_77877; DSSTox_GSID_25670; Imecromone [DCIT]; METHYLUMBELLIFERONE, BETA; WLN: T66 BOVJ E1 IQ; Cholspasmin; Hymechrome; Hymecromonum [INN-Latin]; Himecromona [INN-Spanish]; SMR000471886; 4-Methylumbelliferon [Czech]; 4 Methylumbelliferone; CCRIS 5926; EINECS 201-986-7; BRN 0142217; Himecol; UNII-3T5NG4Q468; AI3-08085; Biliton H; 2H-1-Benzopyran-2-one,7-hydroxy-4-methyl-; Hymecromohe,(S); Cantabiline (TN); 4MU; Hymecromone [USAN:INN:BAN:JAN]; ZZ1; A-Methylumbelliferone; 4-methyl umbelliferone; 4-Methyl-umbelliferone; Coumarin derivative, 3b; HYMECROMONE [MI]; Maybridge1_002078; Prestwick0_000901; Prestwick1_000901; Prestwick2_000901; Prestwick3_000901; HYMECROMONE [INN]; HYMECROMONE [JAN]; Umbelliferone, 4-methyl-; HYMECROMONE [USAN]; 7-Hydroxy-4-methlcoumarin; Methylumbelliferone, .beta.; 4-methyl-7-hydroxy-cumarin; 7-hydroxy-4-methyl-coumari; HYMECROMONE [MART.]; SCHEMBL24150; BSPBio_000742; HYMECROMONE [WHO-DD]; 5-18-01-00439 (Beilstein Handbook Reference); 7-hydroxy-4-methyl coumarine; 79566-13-5; MLS001074671; MLS004491718; 4-Methyl-7-hydroxyl Coumarin; SPBio_002941; 4-Methylumbelliferone - 4-MU; BPBio1_000818; MEGxp0_001898; 7-hydroxy-4-methyl-2-coumarin; 4-Methylumbelliferone, >=98%; DTXSID8025670; ACon1_002401; cid_5280567; HMS547G10; Hymecromone (JP17/USAN/INN); ZINC58121; 7-Hydroxy-4-methyl-2-chromenone; HMS1570F04; HMS2097F04; HMS2267L19; HMS3264E04; HMS3655L16; HMS3714F04; HYMECROMONE [EP MONOGRAPH]; Pharmakon1600-01506174; BCP06770; HY-N0187; Tox21_110385; Tox21_300915; BBL000531; BDBM50022178; CCG-47894; NSC760397; s2256; STK364326; 7-Hydroxy-4-methyl-2H-2-chromenone; AKOS000119370; Tox21_110385_1; 2-Hydroxy-4-methyl-7H-chromen-7-one; AM85958; CS-7560; DB07118; NSC-760397; NCGC00016345-02; NCGC00016345-03; NCGC00016345-04; NCGC00016345-05; NCGC00016345-08; NCGC00016345-09; NCGC00169880-01; NCGC00169880-02; NCGC00257522-01; 7-Hydroxy-4-methyl-2H-chromen-2-one #; AC-22306; AS-13247; NCI60_042099; SY017941; DB-029370; 2-Hydroxy-4-methyl-7H-1-benzopyran-7-one; AB00443536; FT-0602257; FT-0658701; FT-0660634; M0453; SW197287-3; EN300-16753; C03081; D00170; D70647; M-5410; 7-Hydroxy-4-methylcoumarin|4-Methylumbelliferone; AB00443536_08; A843515; Q904431; SR-01000637483; SR-01000637483-1; SR-01000637483-3; BRD-K46424862-001-02-6; BRD-K46424862-001-04-2; Z56763041; F0849-0318; F1918-0038
|
|
| CAS | 90-33-5 | |
| PubChem CID | 5280567 | |
| ChEMBL ID | CHEMBL12208 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 176.17 | ALogp: | 1.9 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 13 | QED Weighted: | 0.627 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.717 | MDCK Permeability: | 0.00001540 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.997 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.952 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.047 | Plasma Protein Binding (PPB): | 89.28% |
| Volume Distribution (VD): | 0.573 | Fu: | 14.30% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.959 | CYP1A2-substrate: | 0.944 |
| CYP2C19-inhibitor: | 0.303 | CYP2C19-substrate: | 0.078 |
| CYP2C9-inhibitor: | 0.076 | CYP2C9-substrate: | 0.906 |
| CYP2D6-inhibitor: | 0.351 | CYP2D6-substrate: | 0.888 |
| CYP3A4-inhibitor: | 0.303 | CYP3A4-substrate: | 0.234 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.153 | Half-life (T1/2): | 0.813 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.084 | Human Hepatotoxicity (H-HT): | 0.104 |
| Drug-inuced Liver Injury (DILI): | 0.648 | AMES Toxicity: | 0.032 |
| Rat Oral Acute Toxicity: | 0.142 | Maximum Recommended Daily Dose: | 0.859 |
| Skin Sensitization: | 0.543 | Carcinogencity: | 0.675 |
| Eye Corrosion: | 0.68 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.233 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000798 | 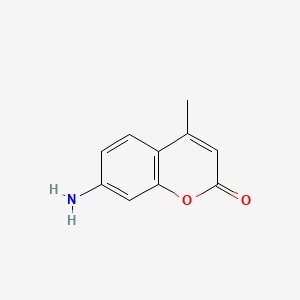 |
0.714 | D0FA2O |  |
0.361 | ||
| ENC001562 | 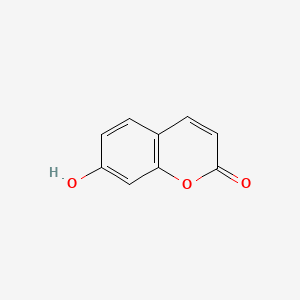 |
0.522 | D04AIT | 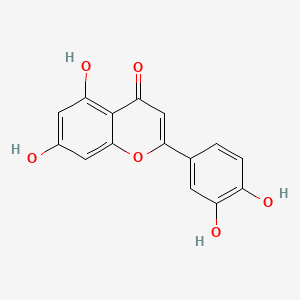 |
0.306 | ||
| ENC002806 | 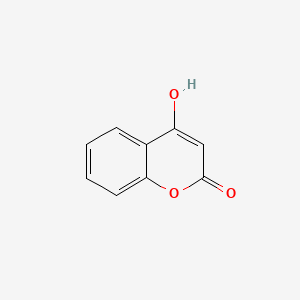 |
0.458 | D0K8KX | 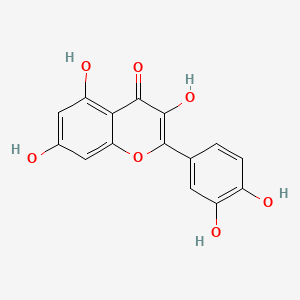 |
0.297 | ||
| ENC001537 |  |
0.442 | D08ZEB |  |
0.288 | ||
| ENC001561 | 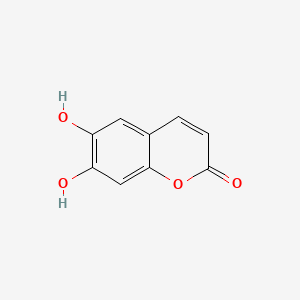 |
0.440 | D08SKH |  |
0.281 | ||
| ENC001393 | 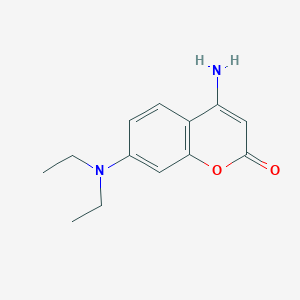 |
0.431 | D0G5UB | 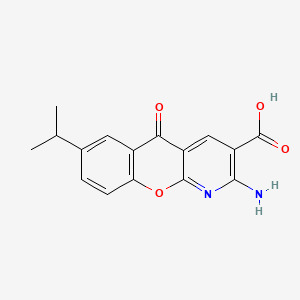 |
0.280 | ||
| ENC002801 |  |
0.429 | D0Z3DY |  |
0.276 | ||
| ENC001617 |  |
0.423 | D0U5QK |  |
0.269 | ||
| ENC005178 |  |
0.423 | D0QV5T |  |
0.263 | ||
| ENC001447 |  |
0.412 | D03GET |  |
0.263 | ||