NPs Basic Information

|
Name |
Naphthalene
|
| Molecular Formula | C10H8 | |
| IUPAC Name* |
naphthalene
|
|
| SMILES |
C1=CC=C2C=CC=CC2=C1
|
|
| InChI |
InChI=1S/C10H8/c1-2-6-10-8-4-3-7-9(10)5-1/h1-8H
|
|
| InChIKey |
UFWIBTONFRDIAS-UHFFFAOYSA-N
|
|
| Synonyms |
naphthalene; 91-20-3; Naphthalin; Tar camphor; White tar; Albocarbon; Naphthene; Camphor tar; Naphthaline; Moth flakes; Moth balls; naphtalene; Dezodorator; Naftalen; Mighty 150; napthalene; Naphthalinum; RCRA waste number U165; Mighty RD1; naftaleno; Mothballs; NCI-C52904; naftalina; naphtaline; naphthalen; NSC 37565; NSC-37565; CHEMBL16293; CHEBI:16482; 2166IN72UN; Naphthalene, 99%; MFCD00001742; NCGC00090793-02; DSSTox_CID_913; DSSTox_RID_75860; DSSTox_GSID_20913; Naftalen [Polish]; Naphthalene, analytical standard; Caswell No. 587; Naphtalinum; Naphthalene [BSI:ISO]; Naphtalene [ISO:French]; Naphthalene, pure; CAS-91-20-3; Naphthalene, molten; CCRIS 1838; HSDB 184; Naphthalene (molten); EINECS 202-049-5; UN1334; UN2304; RCRA waste no. U165; EPA Pesticide Chemical Code 055801; NAPHTHALENE (1,2,3,4,5,6,7,8-D8); Naphthalene, crude or refined; UNII-2166IN72UN; AI3-00278; 2-naphthalen; 1-Naphthalene; 2-Naphthalene; Naphthalene,(S); Naphthalene, 98%; NAPHTHALENE [MI]; NAPHTHALENE [ISO]; NAPHTHALENE [HSDB]; NAPHTHALENE [IARC]; EC 202-049-5; NAPHTHALINUM [HPUS]; NAPHTHALENE [MART.]; NAPHTHALENE [USP-RS]; NAPHTHALENE [WHO-DD]; MLS001055498; WLN: L66J; BIDD:ER0665; DTXSID8020913; HMS3039N15; ZINC967522; AMY22299; NSC37565; Tox21_111023; Tox21_202004; Tox21_300008; BDBM50159249; Naphthalene, for synthesis, 98.5%; STL282720; AKOS000119977; Naphthalene 100 microg/mL in Methanol; UN 1334; UN 2304; Naphthalene 10 microg/mL in Cyclohexane; NCGC00090793-01; NCGC00090793-03; NCGC00090793-04; NCGC00090793-05; NCGC00254058-01; NCGC00259553-01; 68412-25-9; BS-22320; Naphthalene 10 microg/mL in Acetonitrile; SMR000677944; Naphthalene 100 microg/mL in Acetonitrile; Naphthalene, SAJ first grade, >=98.0%; Bicyclo[4.4.0]deca-1,3,5,7,9-pentene; FT-0651884; FT-0672611; FT-0672612; N0004; N0885; EN300-21626; C00829; D97670; Naphthalene, suitable for scintillation, >=99%; L001166; Naphthalene, molten [UN2304] [Flammable solid]; Q179724; SR-01000854997; Melting point standard 79-81C, analytical standard; SR-01000854997-2; F0001-2217; Naphthalene, certified reference material, TraceCERT(R); Z104506008; Naphthalene, crude or refined [UN1334] [Flammable solid]; Naphthalene, United States Pharmacopeia (USP) Reference Standard; Naphthalene, Pharmaceutical Secondary Standard; Certified Reference Material; 25135-16-4; 72931-45-4
|
|
| CAS | 91-20-3 | |
| PubChem CID | 931 | |
| ChEMBL ID | CHEMBL16293 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 128.17 | ALogp: | 3.3 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 10 | QED Weighted: | 0.508 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.237 | MDCK Permeability: | 0.00002340 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.036 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.988 |
| 30% Bioavailability (F30%): | 0.326 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.652 | Plasma Protein Binding (PPB): | 95.69% |
| Volume Distribution (VD): | 1.177 | Fu: | 5.17% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.988 | CYP1A2-substrate: | 0.621 |
| CYP2C19-inhibitor: | 0.819 | CYP2C19-substrate: | 0.246 |
| CYP2C9-inhibitor: | 0.366 | CYP2C9-substrate: | 0.339 |
| CYP2D6-inhibitor: | 0.098 | CYP2D6-substrate: | 0.494 |
| CYP3A4-inhibitor: | 0.024 | CYP3A4-substrate: | 0.249 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.472 | Half-life (T1/2): | 0.399 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.07 | Human Hepatotoxicity (H-HT): | 0.037 |
| Drug-inuced Liver Injury (DILI): | 0.473 | AMES Toxicity: | 0.411 |
| Rat Oral Acute Toxicity: | 0.088 | Maximum Recommended Daily Dose: | 0.045 |
| Skin Sensitization: | 0.887 | Carcinogencity: | 0.796 |
| Eye Corrosion: | 0.912 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.3 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000169 | 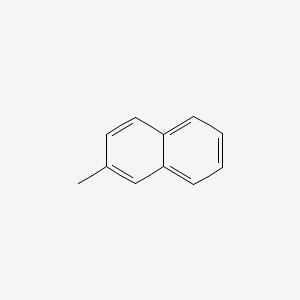 |
0.550 | D0B1FE | 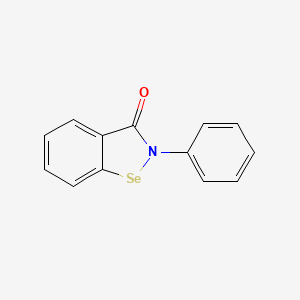 |
0.400 | ||
| ENC000167 | 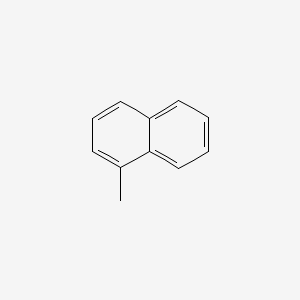 |
0.512 | D0G1VX | 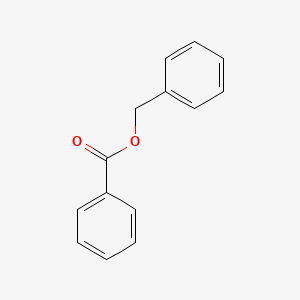 |
0.400 | ||
| ENC000714 |  |
0.512 | D08FTG | 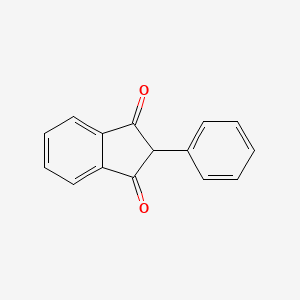 |
0.386 | ||
| ENC000321 |  |
0.500 | D02WCI |  |
0.373 | ||
| ENC000036 |  |
0.468 | D0O6IZ |  |
0.368 | ||
| ENC000159 | 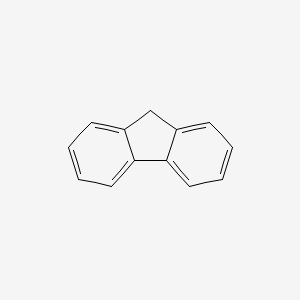 |
0.468 | D04DXN | 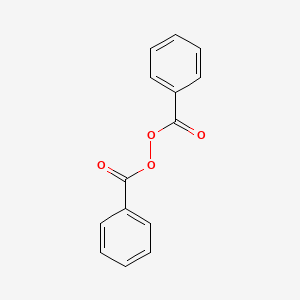 |
0.367 | ||
| ENC000892 |  |
0.462 | D04MSM | 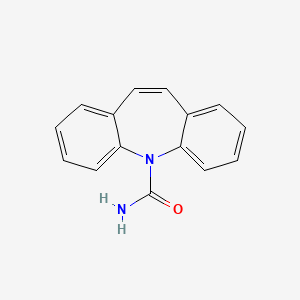 |
0.367 | ||
| ENC000732 |  |
0.453 | D0T5UL |  |
0.367 | ||
| ENC000737 | 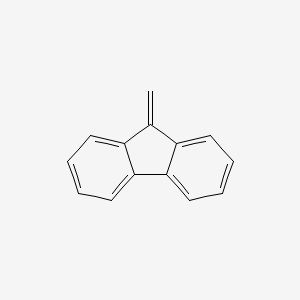 |
0.449 | D0Y0JH |  |
0.361 | ||
| ENC000093 | 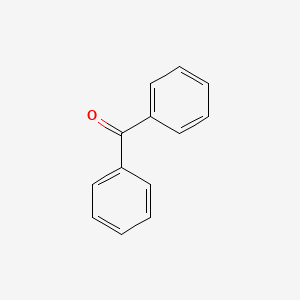 |
0.449 | D00HPK | 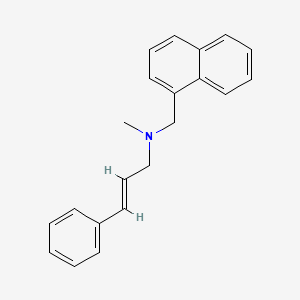 |
0.357 | ||