NPs Basic Information

|
Name |
2-Naphthalenemethanol
|
| Molecular Formula | C11H10O | |
| IUPAC Name* |
naphthalen-2-ylmethanol
|
|
| SMILES |
C1=CC=C2C=C(C=CC2=C1)CO
|
|
| InChI |
InChI=1S/C11H10O/c12-8-9-5-6-10-3-1-2-4-11(10)7-9/h1-7,12H,8H2
|
|
| InChIKey |
MFGWMAAZYZSWMY-UHFFFAOYSA-N
|
|
| Synonyms |
2-NAPHTHALENEMETHANOL; 1592-38-7; naphthalen-2-ylmethanol; 2-Naphthylmethanol; 2-Hydroxymethylnaphthalene; (2-Naphthyl)methanol; 2-Naphthalene methanol; Naphthalen-2-yl-methanol; (naphthalen-2-yl)methanol; beta-Naphthylcarbinol; CHEBI:27615; MFCD00004124; 2-naphthalenearbinol; 2-naphthylmethan-1-ol; 2-naphtylmethanol; Naphthalenemethanol; 2NA; (2-naphtyl)methanol; naphth-2-yl-methanol; NSC 408615; 2-Naphthylmethanol #; naphthalene-2-methanol; naphthalen-2-yl methanol; bmse000683; 2-(hydroxymethyl)napthalene; 2-(Hydroxymethyl)naphthalene; SCHEMBL94384; 2-Naphthalenemethanol, 98%; CHEMBL150445; F3395-0160; DTXSID70166607; ZINC968147; BCP27493; NSC408615; AKOS000249365; AC-5795; CS-W001953; FS-3194; NSC-408615; SY021110; AM20060936; FT-0608823; N0881; C02909; EN300-1263458; 592N387; J-510146; Q27103227
|
|
| CAS | 1592-38-7 | |
| PubChem CID | 74128 | |
| ChEMBL ID | CHEMBL150445 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 158.2 | ALogp: | 2.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 2 |
| Heavy Atoms: | 12 | QED Weighted: | 0.676 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.276 | MDCK Permeability: | 0.00001860 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.96 |
| 30% Bioavailability (F30%): | 0.187 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.897 | Plasma Protein Binding (PPB): | 90.14% |
| Volume Distribution (VD): | 1.485 | Fu: | 11.11% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.971 | CYP1A2-substrate: | 0.388 |
| CYP2C19-inhibitor: | 0.683 | CYP2C19-substrate: | 0.161 |
| CYP2C9-inhibitor: | 0.144 | CYP2C9-substrate: | 0.173 |
| CYP2D6-inhibitor: | 0.395 | CYP2D6-substrate: | 0.838 |
| CYP3A4-inhibitor: | 0.037 | CYP3A4-substrate: | 0.369 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.848 | Half-life (T1/2): | 0.663 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.053 | Human Hepatotoxicity (H-HT): | 0.051 |
| Drug-inuced Liver Injury (DILI): | 0.499 | AMES Toxicity: | 0.229 |
| Rat Oral Acute Toxicity: | 0.029 | Maximum Recommended Daily Dose: | 0.099 |
| Skin Sensitization: | 0.814 | Carcinogencity: | 0.711 |
| Eye Corrosion: | 0.023 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.034 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000169 | 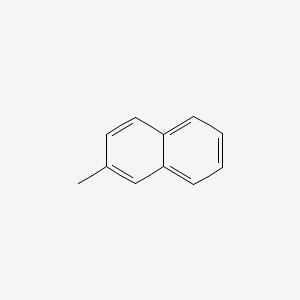 |
0.634 | D05OIS |  |
0.450 | ||
| ENC000047 |  |
0.512 | D0Y7EM | 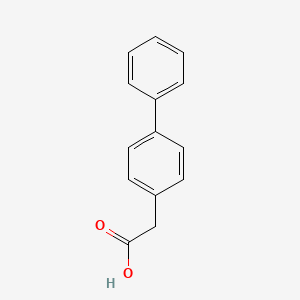 |
0.373 | ||
| ENC000167 | 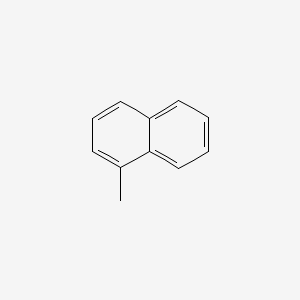 |
0.457 | D0H6TP |  |
0.362 | ||
| ENC000014 |  |
0.450 | D02NTO | 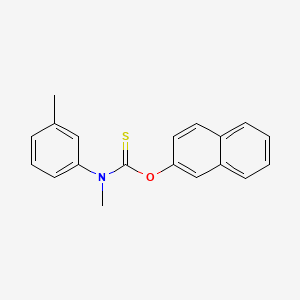 |
0.361 | ||
| ENC001367 | 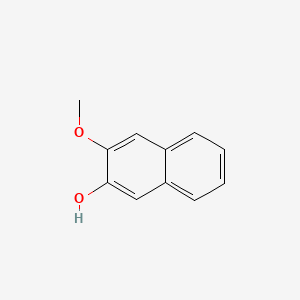 |
0.440 | D0O6IZ |  |
0.361 | ||
| ENC000025 |  |
0.426 | D05CKR |  |
0.361 | ||
| ENC000892 |  |
0.409 | D04JEE |  |
0.348 | ||
| ENC000003 |  |
0.395 | D0L5PO |  |
0.317 | ||
| ENC000683 |  |
0.380 | D02WCI |  |
0.316 | ||
| ENC000041 | 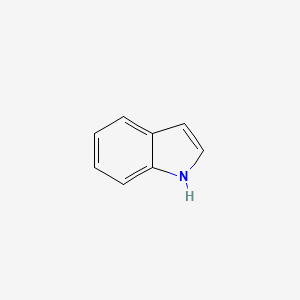 |
0.378 | D0A1PX | 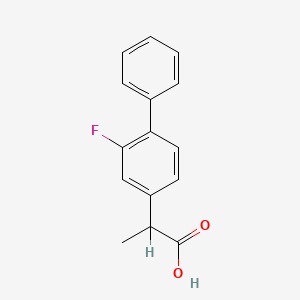 |
0.308 | ||