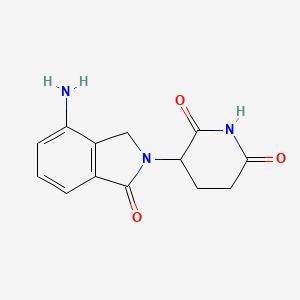
Lenalidomide, 191732-72-6, Revlimid, Revimid, 3-(4-Amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione, CC-5013, CDC 501, CDC-501, Lenalidomide (CC-5013), 3-(4-Amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione, 2,6-Piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-, IMiD3, CC 5013, 3-(7-amino-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione, Lenadoamide, NSC-747972, UNII-F0P408N6V4, DTXSID8046664, CHEBI:63791, ENMD 0997, HSDB 8220, C13H13N3O3, F0P408N6V4, SYP-1512, Revlimid (TN), (3S)-3-(4-Amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione, 1-oxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline, DTXCID6026664, MFCD07772307, NSC 747972, NCGC00167491-01, Lenalidomide [USAN], 3-(4-amino-1-oxo-2,3-dihydro-1H-isoindol-2-yl)piperidine-2,6-dione, LENALIDOMIDE (MART.), LENALIDOMIDE [MART.], Revlimid (lenalidomide), 3-(4-Amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione, IMiD3 cpd, CAS-191732-72-6, Lenalidomide (USAN/INN), lenalidomidum, Lenalidomida, Lenalidomide?, Lenalidomide [USAN:INN:BAN], 443912-14-9, ENMD-0997, IMID-5013, CDC-5013, ALBB-015321, LENALIDOMIDE [MI], LENALIDOMIDE [INN], LENALIDOMIDE [JAN], CHEMBL848, Revlimid (TN) (Celgene), LENALIDOMIDE [VANDF], SCHEMBL32978, MLS003899194, LENALIDOMIDE [WHO-DD], GTPL7331, SCHEMBL1980410, LENALIDOMIDE [EMA EPAR], 3-(4-Amino-1-oxo-2-isoindolinyl)piperidine-2,6-dione, SCHEMBL26646478, BDBM65454, L04AX04, 3-(7-Amino-3-oxo-1H-isoindol-2-yl)-piperidine-2,6-dione, BCPP000186, HMS3654G07, HMS3674C05, LENALIDOMIDE [ORANGE BOOK], BCP01390, HY-A0003, Revlimid, Lenalidomide, CC-5013, Tox21_112492, AC-914, NSC703813, NSC747972, s1029, STK639603, 2,6-Piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2H- isoindol-2-yl)-, AKOS005146276, AKOS005174869, Tox21_112492_1, BCP9000847, CCG-264781, CS-0125, DB00480, KS-1207, SB66166, NCGC00167491-02, NCGC00167491-03, NCGC00167491-04, BL164614, BP-27972, SMR002529986, SY047646, AM20050439, NS00003666, SW218084-2, D04687, EN300-118706, AB01273975-01, AB01273975-02, AB01273975_03, Q425681, SR-01000883999, Q-101410, SR-01000883999-1, 1-Oxo-4-amino-2-(2,6-dioxopiperidin-3-yl)isoindole, Z1515385074, 2, 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-, 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)-2,6-dioxopiperidine, 3-(4-Amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)-2,6-piperidinedione, (3RS)-3-(4-Amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione, 4-amino-2-(6-hydroxy-2-oxo-2,3,4,5-tetrahydropyridin-3-yl)-2,3-dihydro-1H-isoindol-1-one