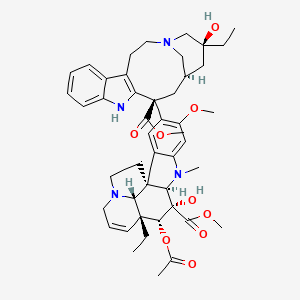
vinblastine, Vinblastin, 865-21-4, Vincaleucoblastin, Vincaleukoblastine, Vinblastina, Vincoblastine, Rozevin, Nincaluicolflastine, Vinblastinum, VR-8, Vinblastinum [INN-Latin], Velban, CCRIS 9002, HSDB 3263, NCI-C04842, UNII-5V9KLZ54CY, EINECS 212-734-0, 5V9KLZ54CY, NSC 47842, NDC 0002-1452-01, NSC-47842, (2ALPHA,2'BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLASTINE, CHEMBL159, DTXSID8021430, NSC 49842, (3aR-(3aalpha,4beta,5beta,5abeta,9(3R*,5S*,7R*,9S*),10bR*,13aalpha))-methyl 4-(acetyloxy)-3a-ethyl-9-(5-ethyl-1,4,5,6,7,8,9,10-octahydro-5-hydroxy-9-(methoxycarbonyl)-2H-3,7-methanoazacycloundecino(5,4-b)indol-9-yl)-3a,4,5,5a,6,11,12,13a-octahydro-5-hydroxy-8-methoxy-6-methyl-1H-indolizino(8,1-cd)carbazole-5-carboxylate, 1H-Indolizino(8,1-cd)carbazole-5-carboxylic acid, 4-(acetyloxy)-3a-ethyl-9-(5-ethyl-1,4,5,6,7,8,9,10-octahydro-5-hydroxy-9-(methoxycarbonyl)-2H-3,7-methanoazacycloundecino(5,4-b)indol-9-yl)-3a,4,5,5a,6,11,12,13a-octahydro-5-hydroxy-8-methoxy-6-methyl-, methyl ester, (3aR-(3aalpha,4beta,5beta,5abeta,9(3R*,5S*,7R*,9S*),10bR*,13aalpha))-, Vinblastina [DCIT], Vinblastinum (INN-Latin), Vinblastine [INN:BAN], methyl (1R,9R,10S,11R,12R,19R)-11-acetyloxy-12-ethyl-4-[(13S,15R,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9-tetraen-13-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate, VLB, VINBLASTINE [INN], cid_5388983, [3H]-Vinblastine, 1z2b, VINBLASTINE [MI], VINBLASTINE [HSDB], VINBLASTINE [VANDF], SCHEMBL3628, BIDD:PXR0201, BSPBio_001228, VINBLASTINE [WHO-DD], DTXCID801430, GTPL6851, L01CA01, 132142-72-4, methyl (3aR,3a1R,4R,5S,5aR,10bR)-4-acetoxy-3a-ethyl-9-((5S,7R,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methano[1]azacycloundecino[5,4-b]indol-9-yl)-5-hydroxy-8-methoxy-6-methyl-3a,3a1,4,5,5a,6,11,12-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate, BDBM50012278, NSC816570, AKOS015965500, CS-1336, DB00570, NSC-816570, NCGC00022585-04, NCGC00022585-05, NCGC00485975-02, 1ST15295, AC-24191, AS-15821, HY-17418, VinblastineVincaleukoblastine; Vinblastina, FT-0699091, EN300-19874057, VINDESINE SULFATE IMPURITY B [EP IMPURITY], VINCRISTINE SULFATE IMPURITY H [EP IMPURITY], BRD-K01188359-001-02-0, BRD-K01188359-065-02-5, (2alpha,2'beta,3alpha,5beta,19beta)-vincaleukoblastine, (2ALPHA,2''''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLASTINE, (3AR-(3AALPHA,4BETA,5BETA,5ABETA,9(3R*,5S*,7R*,9S*),10BR*,13AALPHA))-METHYL 4-(ACETYLOXY)-3A-ETHYL-9-(5-ETHYL-1,4,5,6,7,8,9,10-OCTAHYDRO-5-HYDROXY-9- (METHOXYCARBONYL)-2H-3,7-METHANOAZACYCLOUNDECINO(5,4-B)INDOL-9-YL)- 3A,4,5,5A,6,11,12,13A-OCTAHYDRO-5-HYDROXY-8-METHOXY-6-METHYL-1H-INDOLIZINO (8,1-CD)CARBAZOLE-5-CARBOXYLATE, methyl (1R,9R,10S,11R,12R,19R)-11-(acetyloxy)-12-ethyl-4-[(13S,15R,17S)-17-ethyl-17-hydroxy-13-(methoxycarbonyl)-1,11-diazatetracyclo[13.3.1.0^{4,12}.0^{5,10}]nonadeca-4(12),5(10),6,8-tetraen-13-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.0^{1,9}.0^{2,7}.0^{16,19}]nonadeca-2,4,6,13-tetraene-10-carboxylate, VBL