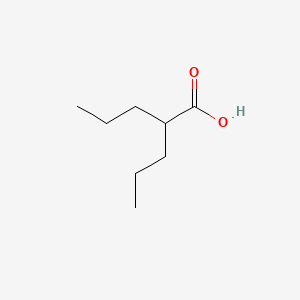
VALPROIC ACID, 2-Propylpentanoic acid, 99-66-1, Dipropylacetic acid, 2-Propylvaleric acid, Depakene, Valproate, Depakine, Mylproin, Ergenyl, Di-n-propylacetic acid, Propylvaleric acid, Pentanoic acid, 2-propyl-, n-Dipropylacetic acid, 4-Heptanecarboxylic acid, Myproic Acid, Depakin, n-DPA, Di-n-propylessigsaure, Dipropylacetate, Convulex, Stavzor, Acido valproico, Acide valproique, Kyselina 2-propylvalerova, Acidum valproicum, 2-n-Propyl-n-valeric acid, Acetic acid, dipropyl-, Valproinsaeure, Avugane, Baceca, 2,2-di-n-propylacetic acid, Abbott 44090, 2-PROPYL-PENTANOIC ACID, Valeric acid, 2-propyl-, Savicol, Depakine chrono, Depakin chrono, Di-n-propylessigsaeure, Acide valproique [INN-French], Acido valproico [INN-Spanish], Acidum valproicum [INN-Latin], Di-n-propylessigsaure [German], Epilim, Kyselina 2-propylvalerova [Czech], VPA, NSC 93819, HSDB 3582, pentanoic acid, 2-propyl, EINECS 202-777-3, VPA;2-Propylpentanoic Acid, UNII-614OI1Z5WI, Valproic acid extended release, BRN 1750447, PEAC, 614OI1Z5WI, DTXSID6023733, CHEBI:39867, AI3-10500, 2 Propylpentanoic Acid, Depakene (TN), MFCD00002672, Vupral, CHEMBL109, (n-C3H7)2CHCOOH, DTXCID803733, EC 202-777-3, NSC93819, Valerin, NSC-93819, Valproic acid [USAN:USP:INN:BAN], NCGC00091149-01, Deproic, Alti-Valproic, Novo-Valproic, Penta-Valproic, Dom-Valproic, Med Valproic, Nu-Valproic, Valproic acid USP, PMS-Valproic Acid, VALPROIC ACID (MART.), VALPROIC ACID [MART.], Acide valproique (INN-French), Acido valproico (INN-Spanish), Acidum valproicum (INN-Latin), VALPROIC ACID (USP-RS), VALPROIC ACID [USP-RS], Valproic acid (USP), VALPROIC ACID (EP IMPURITY), VALPROIC ACID [EP IMPURITY], VALPROIC ACID (EP MONOGRAPH), VALPROIC ACID (USP IMPURITY), VALPROIC ACID [EP MONOGRAPH], VALPROIC ACID [USP IMPURITY], Valproic acid (USAN:USP:INN:BAN), VALPROIC ACID (USP MONOGRAPH), VALPROIC ACID [USP MONOGRAPH], CAS-99-66-1, Depakote (TM), SMR000499581, VALPROICACID, 2 PP (base), Valproinsaure, Valproic, valproic-acid, Novo-divalproex, Sandoz valproic, Dom-valproate, Gen-divalproex, Apo-valproic, APO-divalproex, DOM-divalproex, Epival er, PHL-valproate, PMS-valproate, PMS-Divalproex, Erganyl; Stavzor, Dom-valproic acid, PHL-valproic acid, Epiject I.V., 2-propyl-Pentanoate, Epical (TM), Epilim (Salt/Mix), Depacon (Salt/Mix), Convulex (Salt/Mix), Eurekene (Salt/Mix), G2M-777, Valparin (Salt/Mix), Novo-Valproic - ECC, Spectrum_000521, Divalproex (Salt/Mix), Ratio-Valproic - ECC, Valdisoval (Salt/Mix), 2 -propylpentanoic acid, di-n-propyl acetic acid, S(-)-4-En-valproate, Spectrum2_000946, Spectrum3_001733, Spectrum4_000376, DOM-valproic acid E.C., PHL-valproic acid E.C., PMS-valproic acid E.C., Acidum valproicum (Latin), Novo-valproic soft gel cap, SCHEMBL2275, VALPROIC ACID [MI], VALPROIC ACID [INN], S-2-n-Propyl-4-pentenoate, (S)-2-propyl-4-pentanoate, KBioGR_000871, KBioGR_002277, KBioSS_001001, KBioSS_002278, VALPROIC ACID [HSDB], VALPROIC ACID [USAN], MLS001076682, MLS001335927, MLS001335928, MLS002415770, BIDD:GT0858, DivK1c_000273, VALPROIC ACID [VANDF], SPBio_000912, GTPL7009, VALPROIC ACID [WHO-DD], WLN: QVY3 & 3, KBio1_000273, KBio2_001001, KBio2_002277, KBio2_003569, KBio2_004845, KBio2_006137, KBio2_007413, KBio3_002626, KBio3_002757, NIJJYAXOARWZEE-UHFFFAOYSA-, Valproic acid [USAN:BAN:INN], NINDS_000273, GLXC-02555, HMS2089J06, HMS2231E06, HMS3259C18, HMS3370C21, HMS3715B15, HMS3885G14, Valproic acid, 1mg/ml in Methanol, ALBB-032973, BCP33204, VALPROIC ACID [ORANGE BOOK], Tox21_111091, Tox21_201963, Tox21_300603, BDBM50003616, LMFA01020291, s3944, STL445581, AKOS009156895, Tox21_111091_1, Valproic Acid 1.0 mg/ml in Methanol, CCG-221127, CS-1765, DB00313, NC00584, SDCCGSBI-0050864.P004, NCGC00091149-02, NCGC00091149-03, NCGC00091149-04, NCGC00091149-05, NCGC00091149-06, NCGC00091149-08, NCGC00091149-09, NCGC00091149-21, NCGC00091149-26, NCGC00162288-07, NCGC00254365-01, NCGC00259512-01, AS-11354, HY-10585, SBI-0050864.P003, FT-0609289, FT-0675769, NS00010277, P0823, EN300-64925, A19450, C07185, D00399, AB00698315-06, Q240642, Q-200321, Sodium valproate; 2-Propylpentanoic acid sodium salt, SR-01000075242-7, F2191-0115, Z756391526, Valproic acid, European Pharmacopoeia (EP) Reference Standard, Valproic acid, United States Pharmacopeia (USP) Reference Standard, InChI=1/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10), Valproic acid for system suitability, European Pharmacopoeia (EP) Reference Standard, Valproic acid, Pharmaceutical Secondary Standard; Certified Reference Material