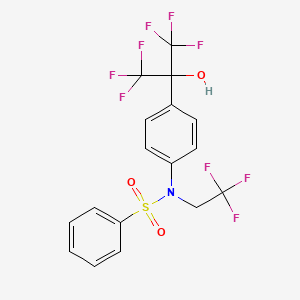
293754-55-9, T0901317, T 0901317, TO-901317, T-0901317, N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide, Benzenesulfonamide, N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-, N-(2,2,2-TRIFLUOROETHYL)-N-{4-[2,2,2-TRIFLUORO-1-HYDROXY-1-(TRIFLUOROMETHYL)ETHYL]PHENYL}BENZENESULFONAMIDE, N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-N-(2,2,2-trifluoroethyl)benzenesulfonamide, TO901317, CHEMBL62136, T-1317, ML125, A07663A39I, N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]benzenesulfonamide, N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxy-propan-2-yl)phenyl)-N-(2,2,2-trifluoroethyl)benzenesulfonamide, [3H]T0901317, SR-05000000453, C17H12F9NO3S, UNII-A07663A39I, 1pqc, BENZENESULFONAMIDE, N-(2,2,2-TRIFLUOROETHYL)-N-(4-(2,2,2-TRIFLUORO-1-HYDROXY-1-(TRIFLUOROMETHYL)ETHYL)PHENYL)-, N-(2,2,2-TRIFLUOROETHYL)-N-(4-(2,2,2-TRIFLUORO-1-HYDROXY-1-(TRIFLUOROMETHYL)ETHYL)PHENYL)BENZENESULFONAMIDE, 2o9i, 4nb6, TO 901317, T 1317, MLS002554297, SCHEMBL457231, GTPL2755, DTXSID6040618, BDBM19993, GLXC-02675, HMS3268P10, HMS3413I08, HMS3649G14, HMS3652B22, HMS3677I08, HMS3886B11, BCP03658, EX-A1278, MFCD03412028, s7076, AKOS017345034, CCG-269558, CS-5951, DB07080, SB19595, NCGC00159555-01, NCGC00159555-02, NCGC00159555-03, NCGC00159555-06, AC-32646, Benzenesulfonamide,N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-, HY-10626, SMR001456586, BCP0726000164, NS00068651, SW203816-2, J1.503.100J, C74506, A876482, J-017492, SR-05000000453-1, SR-05000000453-2, SR-05000000453-5, T-901317, BRD-K23383398-001-01-6, Q27088909, T0901317, >=98%, Benzenesulfonamide,?N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-, N-(2,2,2-Trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide, N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2, 2-trifluoroethyl)benzenesulfonamide